At present, the gold standard for the diagnosis of SARS-CoV-2 infection is detection of viral RNA by means of real-time polymerase chain reaction (rt-PCR) testing or an equivalent molecular technique. In Spain, rapid antigen testing (RAT) that is duly validated (sensitivity ≥80% and specificity ≥97%) can be used within five days of the onset of symptoms in patients with no major immunosuppression and no criteria for intensive care unit (ICU) admission1. RAT is less sensitive than rt-PCR testing in all stages of the infection, and even less so in asymptomatic cases, but its use in the absence of symptoms has not been ruled out in all cases1–3. A recent report from the European Centre for Disease Prevention and Control (ECDC) recommends the use of RAT in patients with or without symptoms if a rate of positive tests ≥10% is anticipated2. It also advises its use in high-risk settings to quickly identify infected individuals and implement prevention and control measures to curb transmission, though it does recommend that negative cases be confirmed with rt-PCR testing1–3.
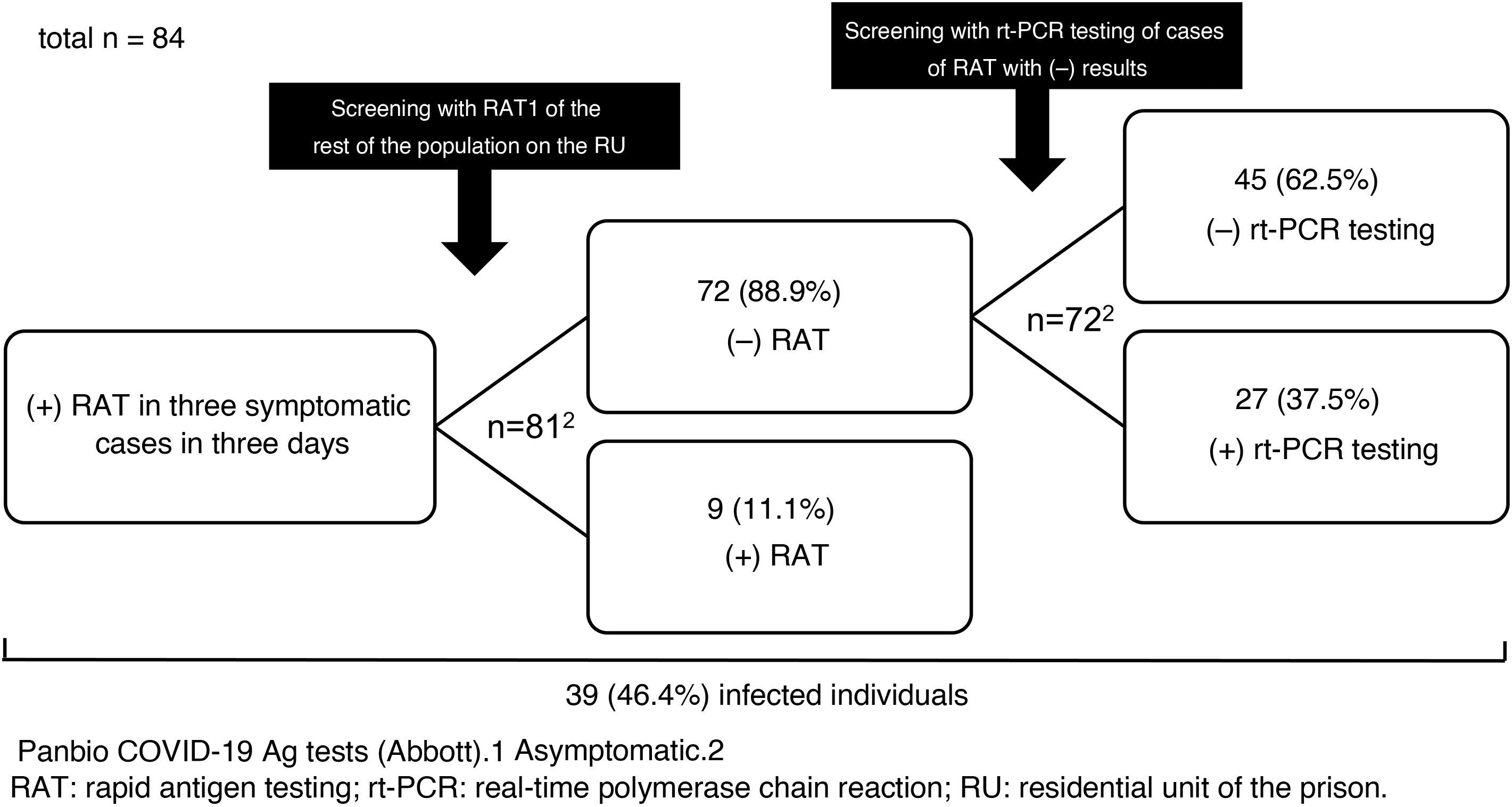
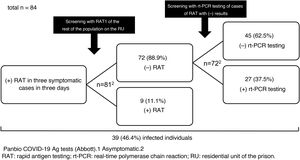
We report the results of the use of RAT in a SARS-CoV-2 outbreak that occurred on a residential unit (RU) of Figueras prison in Girona, Spain, in late 2020. Between 23 and 25 December, SARS-CoV-2 infection was diagnosed by RAT (Panbio™ COVID-19 Ag tests, Abbott) in three inmates with mild respiratory symptoms. The RU was isolated, and on the afternoon of 25 December the rest of the population was screened with RAT (n = 81). Nine inmates (11.1%) tested positive. They were separated from the others, isolation measures were kept in place and the RU was considered a low-complexity COVID unit given the number of asymptomatic and mildly symptomatic cases with no criteria for hospital admission. The unit was equipped with organisational and functional resources to ensure care safety, quality and efficiency. Cleaning, laundry, waste management and distribution of food and medication was organised according to the recommendations of the Catalan Health Department4. The following were indicated: a) strict isolation of the unit with essential healthcare and non-healthcare personnel entering and exiting; b) mandatory use of personal protective equipment (PPE); and c) clinical examinations (oxygen saturation, temperature and questions about the possible onset of symptoms) twice daily.
On 28 December, rt-PCR testing was performed in the 72 cases with previously negative RAT, yielding positive results in 27 (37.5%) of them (Fig. 1). All followed a good clinical course and there were no hospital admissions.
The prevalence of infection (46.4%) was high as the outbreak occurred in an enclosed space. In situations of confinement, it is estimated that the contagion rate (R0: mean number of people infected by an infected person) may be five to 14 times higher than usual (normally, 1.5–3.0)5; this explains the high number of infections detected in the outbreak. The measures adopted were satisfactory and rt-PCR testing results were negative in all contacts at seven and 14 days.
Regarding the use of RAT in asymptomatic close contacts, some studies (an original6, a letter to the editor7 and several preprints8–10) have shown it to have a specificity equal or close to 100%7–10, but a much lower sensitivity, between 33% and 66%6–10. None of these studies was conducted in contacts from an outbreak or in confined groups. In the cases on the RU, the sensitivity was 25% and the negative predictive value, a key indicator in scenarios in which the prevalence can be considered moderate or high, was 63%. Although RAT is appealing as it is a quick and easy technique that does not require qualified operators, the risk of false negatives is high, even in an outbreak in a confined space with a high prevalence of positive results such as the one reported. Consequently, rt-PCR testing should be the test of choice in screening asymptomatic patients. Only if rt-PCR test results cannot be obtained quickly and there is a high risk of transmission could initial screening with RAT be advisable. In those cases, negative results should be confirmed with subsequent rt-PCR testing, as can be deduced from this study and as is suggested by the guidelines and protocols from the Spanish Ministry of Health1, the ECDC2 and the United States Centers for Disease Control and Prevention (CDC)3.
FundingThe authors declare that they received no funding to conduct this study.
Please cite this article as: Marco A, Solé C, Abdo IJ, Turu E. Baja sensibilidad de los test rápidos antigénicos como método de cribado en un brote de infección por SARS-CoV-2 en prisión. Enferm Infecc Microbiol Clin. 2022;40:152–154.