The use of extracorporeal membrane oxygenation (ECMO) life support systems has increased in recent years. They can be colonized by several microorganisms, particularly fungi, giving rise to nosocomial infections, such as invasive fungal infection (IFI), in the form of central line-associated bloodstream infection (CLABSI) and circuit infection. These microorganisms, moreover, can develop a biofilm that perpetuates their existence.
When IFI is confirmed in a patient on ECMO, the circuit should be changed or even removed, depending on the patient's condition. The use of antifungal drugs with activity against biofilm could allow IFI treatment to continue without removing the circuit.
We present the case of an infant on ECMO support who suffered a CLABSI when the circuit was infected by Candida. The patient was treated with micafungin without ECMO being changed or removed.
A female infant of 20 months of age, weighting 12kg, with a body mass index of 0.6, with no relevant personal or family history, was admitted to the pediatric intensive care unit (PICU), following referral from another center to receive ECMO support. Diagnosis was necrotizing pneumonia by Streptococcus pneumoniae, with acute respiratory distress syndrome and septic shock.
Initial symptoms on admission were a 5-day history of fever up to 40°C, accompanied by respiratory and heart failure.
Community-acquired left lobar pneumonia was diagnosed, with ipsilateral pleural effusion and septic shock, requiring intubation and mechanical ventilation. Broad-spectrum antimicrobial treatment was started with cefotaxime and vancomycin. Vancomycin was withdrawn following isolation of cephalosporine-susceptible serotype 7F/A from culture.
After day 6, her respiratory symptoms worsened, with pneumothorax, which was drained, and serious respiratory destabilization. As a result, high-frequency ventilation was started, with poor response. A new broad-spectrum antimicrobial treatment was started with piperacillin-tazobactam and amikacin, due to the possibility of ventilator-associated pneumonia, and inotropic support (consisting of dopamine up to 16mcg/kg/min and adrenaline up to 0.8mcg/kg/min) was required.
On day 7, due to poor evolution and response to treatment given so far, she was transferred to our PICU. Considering the seriousness of the patient, who had a Pediatric Risk Score of Mortality III (PRISM-III) of 25, oxygenation index of 38, an alveolar arterial difference of 590, 15mmol/L lactate and echocardiographic signs of severe pulmonary hypertension (60–70mmHg), ECMO was started.
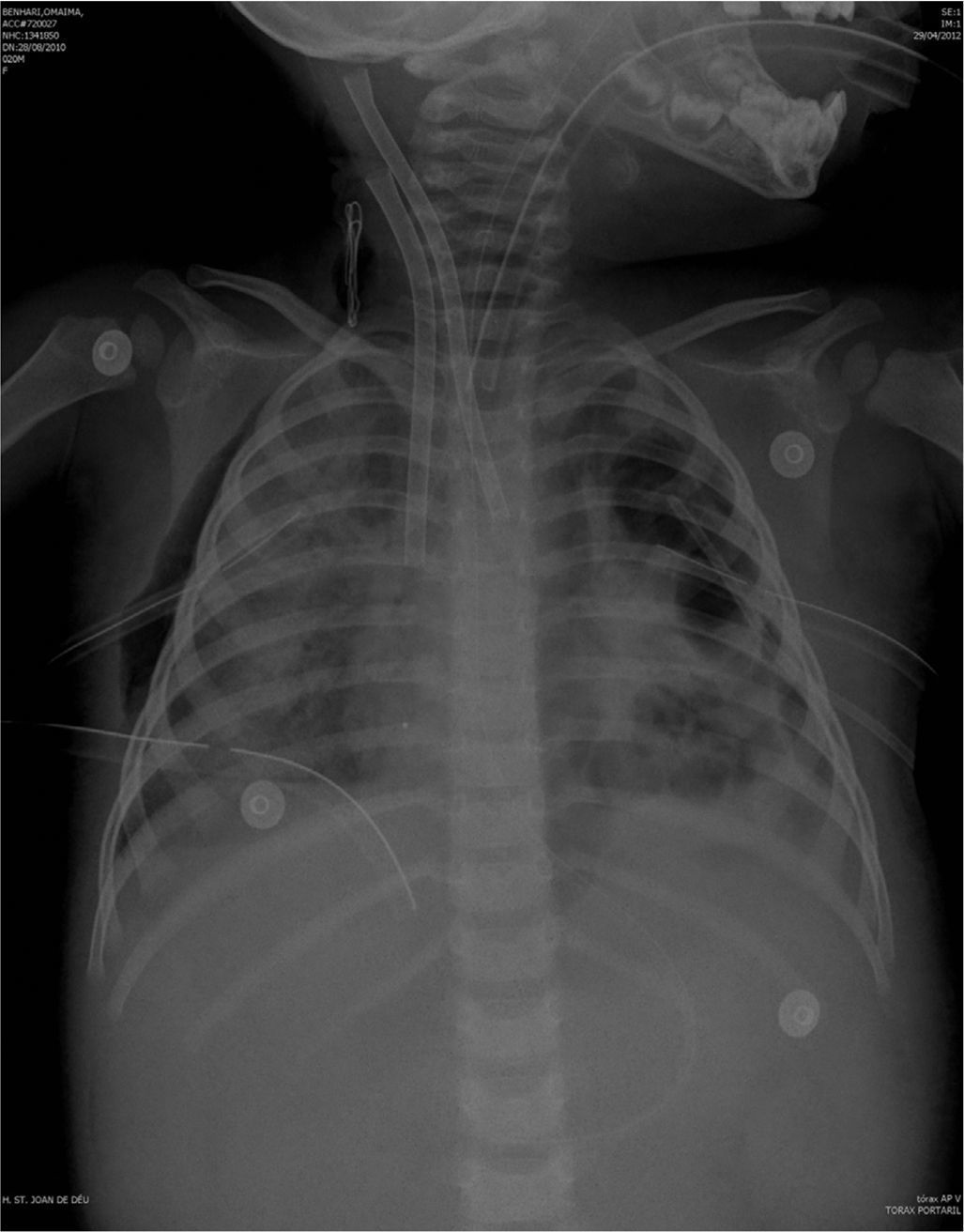
The previously started inotropic therapy was maintained, and a steroid treatment for relative suprarenal insufficiency in the context of septic shock was started. Other treatments were sedation with fentanyl and midazolam, neuromuscular blockade and parenteral nutrition with gastric protection. Fig. 1 shows radiologic examination at the time of starting ECMO.
The patient made good progress, with removal of inotropic support after 4h on ECMO. Respiration also improved, and she was gradually weaned from ventilator support, with radiologic and analytical improvements.
However, after 5 days after admission to our PICU, the patient's condition deteriorated, with febricula and altered analytical parameters (leukocytosis 16,000l/mm3, C-reactive protein 148.7mg/L and procalcitonin 1.5ng/mL, normal lactate). Several cultures were taken from the patient (blood, urine and bronchoalveolar lavage fluid) and from the ECMO circuit connections, and micafungin (4mg/kg/day) was added to previous antibiotic treatment.
At 24 and 72h, Candida tropicallis was found in cultures from day 1 and 3 post IFI (from all patient samples and ECMO circuit culture), with the microorganism proving susceptible to all antifungal drugs tested (fluconazole, itraconazole, voriconazole, amphotericin B, caspofungin and micafungin).
Owing to improvement of clinical and analytic parameters, we decided to watch and wait. Cultures taken at day 5 and 7 post IFI were negative. Studies to evaluate the spread of IFI were also negative (fundoscopy, echocardiogram and abdominal ultrasound).
The patient evolved favorably, with removal of ECMO support 12 days after admission to PICU, and extubation after 15 days.
The antibiotic therapy lasted 7 days in the case of piperacillin-tazobactam, 12 days for amikacin and 12 days for vancomycin. IFI was treated with micafungin during the 12 days the patient was on ECMO, following which it was de-escalated to fluconazole, which was maintained for a further 7 days. Micafungin therapeutic levels were not monitored because in this moment the technique was not available.
The patient was discharged from hospital with no complications.
According to clinical practice guidelines, such as IDSA,1 empirical treatment of IFI depends on the clinical condition of the patient.
In our case, administration of echinocandins was chosen due to patient severity and the anti-biofilm action of the drug, an important factor to consider in patients ECMO support.
Among anti-fungal drugs and among echinocandins, micafungin seems to be the most effective against the biofilm caused by Candida spp, as shown by Tawara,2 by Jacobson3 and by Cateau.4 Fluconazole is commonly indicated for pediatric patients, although in ECMO cases higher doses are needed because of the increase in the volume of distribution. For prophylaxis, the fluconazole recommended dosage is 25mg/kg weekly, but even doses of 30–40mg/kg may be needed for treatment.5
At a clinical level, the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) recommends that the catheter should be removed in catheter-related Candida infections.6 In contrast, the study by Nucci questioned the need to remove the central catheter in patients with CLABSI by Candida,7 since overall results show no significant benefit regarding fungal eradication, recurrence or survival in patients with early catheter removal. The authors hypothesize that the lack of benefit from catheter removal could be due to the antibiofilm activity of the antifungal treatment received (micafungin, caspofungin or liposomal amphotericin B). Ramage et al. recently summarized the significant role of Candida biofilm in infections and its difficult diagnosis and management. In addition to catheter removal, antifungal lock therapy (even with ethanol) and antifungal drugs with antibiofilm activity are recommended for Candida biofilm infections.8
The case presented here supports earlier findings that catheter removal may be avoided in some cases when highly anti-biofilm active drug, such as micafungin, are administered.
Conflict of interestNothing declared.