Crimean-Congo hemorrhagic fever (CCHF) is a viral disease, mainly transmitted through tick bite, of great importance in Public Health. In Spain, Crimean-Congo hemorrhagic fever virus (CCHFV) was detected for the first time in 2010 in Hyalomma lusitanicum ticks collected from deer in Cáceres. The aim of this study was to investigate the presence of CCHFV in ticks from Cáceres, and from other Spanish areas, and to evaluate the presence of antibodies against the virus in individuals exposed to tick bites.
MethodsA total of 2053 ticks (1333 Hyalomma marginatum, 680 H. lusitanicum and 40 Rhipicephalus bursa) were analyzed using molecular biology techniques (PCR) for CCHFV detection. The determination of specific IgG antibodies against CCHFV in 228 serum samples from humans with regular contact with ticks (at risk of acquiring the infection) was performed by indirect immunofluorescence assay.
ResultsThe CCHFV was not amplified in ticks, nor were antibodies against the virus found in the serum samples analyzed.
ConclusionThe absence of the CCHFV in the ticks studied and the lack of antibodies against the virus in individuals exposed to tick bites would seem to suggest a low risk of acquisition of human infection by CCHFV in Spain.
La fiebre hemorrágica de Crimea-Congo (FHCC) es una enfermedad vírica, transmitida fundamentalmente por garrapatas, con gran importancia en salud pública. La primera detección del virus de la fiebre hemorrágica de Crimea-Congo (vFHCC) en España se realizó en 2010 en garrapatas de la especie Hyalomma lusitanicum recogidas sobre ciervos en Cáceres. El objetivo de este estudio fue investigar la presencia del vFHCC en garrapatas de esta y otras zonas de España y evaluar la presencia de anticuerpos frente al virus en personas expuestas a picaduras de garrapatas.
MétodosSe analizaron un total de 2.053 garrapatas (1.333 Hyalomma marginatum, 680 Hyalomma lusitanicum y 40 Rhipicephalus bursa) mediante técnicas de biología molecular (PCR) para la detección del vFHCC. Se realizó la determinación de anticuerpos IgG específicos frente al vFHCC mediante inmunofluorescencia indirecta a 228 sueros de personas en contacto habitual con garrapatas, y por tanto, en riesgo de adquirir la infección.
ResultadosNo se amplificó el vFHCC en garrapatas ni se encontraron anticuerpos frente al mismo en los sueros analizados.
ConclusiónLa ausencia del vFHCC en las garrapatas estudiadas y de anticuerpos frente al mismo en personas expuestas a picaduras indica un riesgo bajo de adquisición de la infección por el vFHCC en humanos en España.
Crimean-Congo hemorrhagic fever virus (CCHFV) is responsible for a human severe hemorrhagic disease, Crimean-Congo hemorrhagic fever (CCHF), transmitted via tick bite or by contact with infected body fluids.1,2 After an incubation period (2–7 days), the disease courses with non-specific symptoms (headache, high fever, myalgia, stomach pain, diarrhea and vomiting) in a period known as prehemorrhagic. This phase is followed by a hemorrhagic period characterized by ecchymosis and petechiae of skin and mucous membranes, epistaxis, gastrointestinal bleeding (haematemesis, melenas, etc.) and severe thrombocytopenia, leukopenia and the elevation of liver enzymes.1–3 The documented fatality rates can reach more than 50%.4 CCHF has a broad world distribution including Africa, Asia and Europe, and it is emerging and reemerging in several countries.1–5 The disease only occurs in humans, but the zoonotic life cycle of the virus includes ticks as vectors and reservoirs (mainly within Hyalomma genus), and animals as amplifiers of the infectious agent or dispersers of the vectors.1,6
The occurrence of the CCHFV was not known in Southern Europe before 2010 when the virus was amplified in Hyalomma lusitanicum ticks captured from deer in Spain (Cáceres, 39.63°N; 7.33°W) by our team.7 The genetic sequence of this virus showed high similarity with strains from Senegal and Mauritania, and it was different from European or Asian strains. Therefore, the potential arrival of infected ticks transported by migratory birds from Africa was hypothesized,7 and our hypothesis was supported by the detection of a similar strain of CCHFV in Morocco, in Hyalomma marginatum ticks removed from migratory birds able to reach the Iberian Peninsula.6
The detection of CCHFV in ticks from Spanish deer and from Moroccan migratory birds, in addition to the presence of H. marginatum established populations (an anthropophilic tick species) and humans susceptible to develop FHCC infections, made necessary to establish a surveillance program of the CCHF in Spain. The present study, supported by the Instituto de Salud Carlos III (Fondo de Investigación Sanitaria, Ministerio de Ciencia e Innovación, Spain; PI12/02579), aimed to investigate the presence of CCHFV in ticks and to screen human antibodies against the virus in Spain.
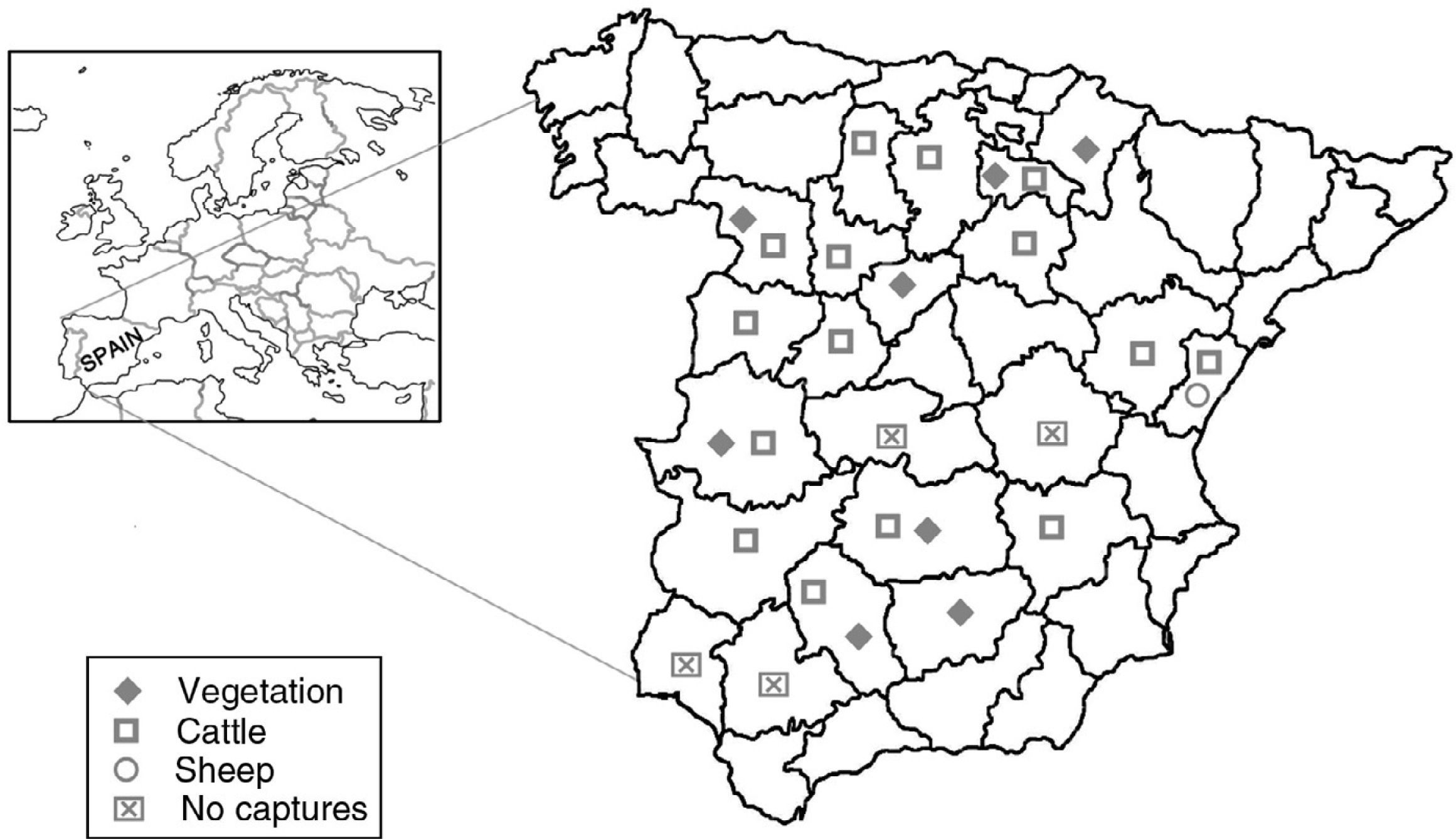
Material and methodsTick collectionFrom 2013 to 2015, ticks were collected from vegetation using dragging methods and from hosts (cattle and sheep) by direct capture. These techniques aimed to obtain the maximum number of Hyalomma spp. specimens, the known vector of the CCHFV. Therefore, samplings were carried out in the optimal season and areas, always taking into account the distribution and the biology of this genus.8 Some Rhipicephalus bursa specimens captured close to the area where the virus had been previously detected7 were also added to the tick collection. A total of 22 Spanish provinces were screened for ticks with the invaluable help of multidisciplinary colleagues (Heads of Public Health Organizations, veterinarians, biologists, forest workers and ranchers).
The morphological classification of ticks was performed using conventional keys and reprints.8,9 This classification was genetically confirmed in doubtful specimens using a PCR targeting a fragment gene of the mitochondrial 16S rRNA,10 as it has been described in previous studies.11 Ticks were kept frozen at −80°C.
Sera collectionDuring 2014 sera samples from volunteers in risk for tick bites or animal contact were collected in Cáceres (7–40km far away from the area where there was evidence of the virus) through the Health Centers from Santiago de Alcántara (31.61°N; 7.2°W), Cedillo (39.65°N; 7.5°W), Carbajo (39.60°N; 7.20°W), Membrío (39.53°N; 7.06°W), Salorino (39.48°N; 7.01°W), and Herreruela (39.46°N; 6.9°W). Sera samples from humans routinely exposed to tick-bites (foresters and forest rangers) in La Rioja (Spain), and from patients with microbiological confirmation of tick-borne disease that attended the Infectious Diseases Department from Hospital San Pedro (which attends all 300,000 inhabitants of La Rioja region) from 2010 to 2014 were also included. All participants were informed of the purpose of the study and a consent was obtained from all of them. This study was performed with the ethical approval of the Committee for Ethics in Clinical Research in La Rioja (Ref. CEICLAR 109 Bis).
CCHFV detectionTicks were washed in 70% ethanol and rinsed twice with sterilized MQ water. A half of each tick was homogenized using the Bullet Blender™ system and 6mm glass balls (maximum speed during 2min). The RNA was extracted from the homogenates using the RNeasy Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's instructions. A negative control without tick was extracted in each batch of extractions. The RNA was retrotranscribed using the Omniscript RT kit (Qiagen, Hilden, Germany). The RNA and cDNA were frozen at −80°C and −20°C, respectively, until their use. The cDNAs were pooled (from 6 to 11 samples per pool, except for 3 ticks from sheep that were processed in one pool) by tick species, gender, source of capture (vegetation or type of host) and area.
The pooled cDNAs were used as templates for PCR assays targeting the 16S mitochondrial RNA10 as internal control to validate the extraction and retrotranscription of the RNA. The CCHFV detection was performed using 4 conventional PCRs (3 nested and 1 single reaction) and a real time assay (qPCR) that amplify fragment genes of the S segment.12–15 A positive control of the virus (AP92 strain) as well as negative controls of extraction and negative controls of PCR were included.
CCHF antibodies detectionThe determination of IgG antibodies against CCHFV was carried out by indirect immunofluorescence assay with the commercial kit “Crimean-Congo Fever Virus Mosaic 2” (Euroimmun AG, Luebeck, Germany), following the manufacturer's instructions.
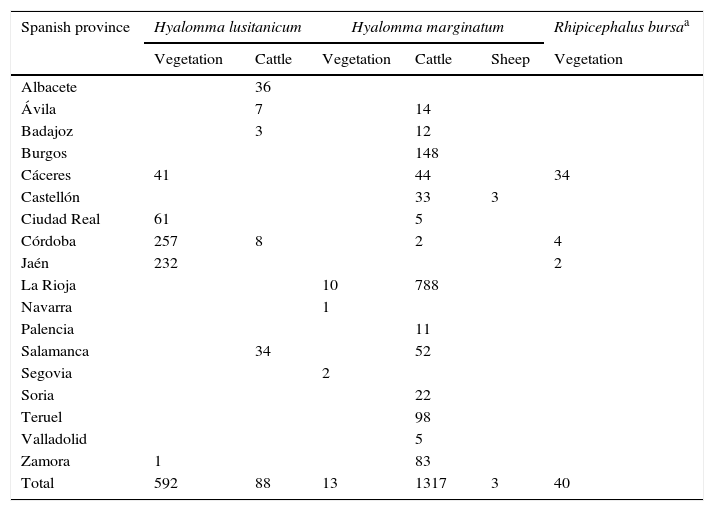
ResultsA total of 2053 adult ticks from 18 Spanish provinces were screened for the CCHFV detection; 1333 H. marginatum, 680 H. lusitanicum and 40 R. bursa specimens (Table 1 and Fig. 1). The cDNAs from tick specimens were grouped in 74 pools from H. lusitanicum, 151 from H. marginatum, and 4 from R. bursa. No samples yielded positive results for the PCRs used for the CCHFV detection.
Tick samples analyzed in this study.
| Spanish province | Hyalomma lusitanicum | Hyalomma marginatum | Rhipicephalus bursaa | |||
|---|---|---|---|---|---|---|
| Vegetation | Cattle | Vegetation | Cattle | Sheep | Vegetation | |
| Albacete | 36 | |||||
| Ávila | 7 | 14 | ||||
| Badajoz | 3 | 12 | ||||
| Burgos | 148 | |||||
| Cáceres | 41 | 44 | 34 | |||
| Castellón | 33 | 3 | ||||
| Ciudad Real | 61 | 5 | ||||
| Córdoba | 257 | 8 | 2 | 4 | ||
| Jaén | 232 | 2 | ||||
| La Rioja | 10 | 788 | ||||
| Navarra | 1 | |||||
| Palencia | 11 | |||||
| Salamanca | 34 | 52 | ||||
| Segovia | 2 | |||||
| Soria | 22 | |||||
| Teruel | 98 | |||||
| Valladolid | 5 | |||||
| Zamora | 1 | 83 | ||||
| Total | 592 | 88 | 13 | 1317 | 3 | 40 |
The detection of the CCHFV in this tick species has been reported, although its role as vector has not been demonstrated.1
One hundred and fourteen human sera specimens collected in Cáceres (54 from Santiago de Alcántara, 16 from Cedillo, 15 from Carbajo, 12 from Membrío, 10 from Salorino and 7 from Herreruela) were analyzed. From them, 97% volunteers had usual contact with animals, 61% were aware of having suffered a tick-bite, and 5% were conscious of having suffered a tick-borne disease. The same number of human sera (n=114) from La Rioja region were also processed: 42 from foresters and forest rangers and 72 from patients with a confirmed tick-borne disease. A total of 70% lived and/or worked in rural areas and they had regular contact with animals, and 67% recalled tick-bites. IgG antibodies against CCHFV were not found in any of the sera samples analyzed.
DiscussionPrevious studies carried out in the Center of Rickettsiosis and Arthropod-borne Diseases in La Rioja demonstrated the circulation of the CCHFV in H. lusitanicum ticks from Cáceres province and the possible arrival of this virus via migratory birds to Spain.6,7 These facts, in addition to the presence of H. marginatum populations that bite people, made us to be aware of the possible occurrence of autochthonous CCHF human cases in Spain. In fact, when we were writing this paper, the first CCHF case has been diagnosed in our country.16–18 Moreover, a second case of a nurse who treated the first patient has been reported.16–18 In the present work, the absence of CCHFV in the ticks analyzed and the lack of antibodies against CCHFV in human populations with high risk of exposition to tick-bites, suggest that, to date, the virus is not widespread and the risk of acquiring the infection and the disease in Spain is low.
Our negative results in ticks suggest a low prevalence of the FHCCV in the vectors, supported also by previous studies carried out in ticks removed from patients, vegetation and hosts (cattle and birds) in the North of Spain.11,19 Moreover, the virus has been repeatedly detected in the same tick species (H. lusitanicum) and from the same area where it was found for the first time (Cáceres).16 The reported rates of the virus infection in ticks from endemic European areas are highly variable (0 to ≥50%).20,21 These differences support that the screening of CCHFV in ticks should continue throughout Spain.
Despite the recent autochthonous CCHF case in Spain, our negative seroepidemiological results were not unexpected since the circulation of the virus in this area seems very recent and not widely distributed. Moreover, the reported seroprevalences of IgG antibodies against CCHFV in populations from the endemic areas showed significant differences, even in different regions of the same country.20,22 The occurrence of the virus in Spain and the recent appearance of the first human case in our country make necessary to continue monitoring the presence of antibodies against CCHFV in more sera samples and areas of the Iberian Peninsula. In addition, a seroepidemiological survey of CCHF in domestic and wild animals is also recommended.
FundingThis work was partly supported by a grant from the Instituto de Salud Carlos III (Fondo de Investigación Sanitaria, Ministerio de Ciencia e Innovación, Spain; PI12/02579) and the European Regional Development Fund (ERDF). Fundación Rioja Salud (Spain) awarded a grant to A.M.P. (FRS/PIF-01/10).
Conflict of interestThe authors declare that they have no conflict of interest.
Partial results from this study were presented at the 19th congress of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC for its acronym in Spanish) held in Seville (Spain) in May 2015.
The authors thank Rufino Álamo (Department of Health of the Junta de Castilla y León, Valladolid); Gerardo Domínguez (Territorial Health Service and Social Welfare of the Junta de Castilla y León, Burgos); Jesús Lanchas (Territorial Health Service and Social Welfare of the Junta de Castilla y León, Salamanca); Manuel Martín (Clinical veterinarian, Zamora and Salamanca), Jesús Cavero (COTEVE S.L., Teruel); Javier Balado (Clinical veterinarian, Castellón); and, Ramón Núñez and Juan Carlos Hernández (Servivet C.D., Ávila) for supplying ticks. The authors also thank Mónica Venturini (Alergology Department, Hospital San Pedro, La Rioja); Isabel González (Health Center of Herreruela, Cáceres); Antonio Santos (Health Center of Salorino, Cáceres); Mª del Mar Carrero (Health Center of Membrío, Cáceres); Juan José Galán (Health Center of Cedillo); Mª Luz Serrano (Health Center of Carbajo, Cáceres); and, María Inocencia Tejero, Mª Gloria Paredes, José Luis Tello, Blanca Pina and María José Rubio (Health Center of Santiago de Alcántara, Cáceres), for providing serum samples. We also thank to Prof. Dr Matthias Niedrig and Dr Oliver Donoso-Mantke (Robert Koch Institute, Germany) for supplying CCHFV positive control.