Outbreaks of human leishmaniasis are not very common in Spain, despite being considered an endemic disease. In this study, a new outbreak in the Valencian Community is presented. Its principal objective is to describe the clinical-epidemiological characteristics and to present the main Public Health measures established to contain the outbreak.
MethodsA case was defined as anyone residing in the village of Tous (in Valencian Community), diagnosed with leishmaniasis according to clinical and laboratory criteria, defined by the Epidemiological Surveillance Network, and date of symptoms onset between 1 January 2012 and 31 December 2013. A descriptive analysis was performed on the epidemiological variables collected.
ResultsA total of 19 cases were reported from the 28th week of 2012 to the 30th week of 2013. Adults were most affected by the disease (73%). The most common clinical form was cutaneous leishmaniasis (89%). More than three-quarters (79%) of cases were confirmed by PCR. Species typing was performed on seven samples, with the parasite Leishmania infantum being identified. Patient outcome was favourable after physical (31%), or pharmacological (69%) treatment. Some kind of residual damage was observed in 37% of cases. Different measures were applied, aimed at health professionals, and vector and reservoir control, as well as general recommendations to the population for the containment of the outbreak.
ConclusionsThis cutaneous leishmaniasis outbreak confirms the endemic nature and the high prevalence of the disease in the Mediterranean area. The most commonly used treatment was intralesional meglumine antimoniate. A comprehensive plan of action had to be developed in order to control the outbreak.
Los brotes de leishmaniasis humana no son muy frecuentes en España, a pesar de ser considerada una enfermedad con carácter endémico. A través del presente estudio se comunica un brote nuevo detectado en la Comunidad Valenciana, con el objetivo principal de describir las características clinicoepidemiológicas y de referir las principales medidas, en materia de Salud Pública, instauradas para la contención del brote.
MétodosSe consideró caso a toda persona con domicilio en la localidad de Tous (en la Comunidad Valenciana), diagnóstico de leishmaniasis según los criterios clínicos y de laboratorio definidos por la Red de Vigilancia Epidemiológica, y fecha de inicio de síntomas entre el 1 de enero de 2012 y el 31 de diciembre de 2015. Se realizó un análisis descriptivo de las distintas variables epidemiológicas recogidas.
ResultadosDesde la semana 28.a del año 2012 hasta la semana 30.a del 2013 se notificaron un total de 19 casos. La edad adulta fue la más afectada (73%). La forma clínica más frecuente fue la leishmaniasis de tipo cutáneo (89%). Un 79% de los casos se confirmaron mediante técnica de PCR; en 7 muestras pudo realizarse la tipificación, siendo el parásito identificado como Leishmania infantum. La evolución de los pacientes fue favorable tras el tratamiento físico (31%) o farmacológico (69%). El 37% presentaron algún tipo de lesión residual. Se aplicaron diversas medidas dirigidas a los profesionales sanitarios y al control del vector y del reservorio, así como recomendaciones generales a la población para la contención del brote.
ConclusionesEste brote de leishmaniasis cutánea confirma el carácter endémico y la elevada prevalencia de la enfermedad en la cuenca mediterránea. El tratamiento más utilizado fue el antimoniato de meglumina intralesional. Para el control del brote fue necesario desarrollar un plan integral de actuación.
Leishmaniasis is a disease produced by a heterogeneous group of protozoa belonging to the genus Leishmania (Trypanosomatidae family) that is transmitted by the bite of an infected female fly that acts as its vector (of the genuses Lutzomyia in the New World or Phlebotomus in the Old World). Several mammals, both wild and domestic, act as reservoirs (canidae, rodents and marsupials), although in the anthroponotic forms, which are most prevalent in India and Sudan, humans are the main reservoir of the disease.1
Clinical manifestations range from skin ulcers to cases with multi-organ systemic disease. The cutaneous form of leishmaniasis has different expression patterns, although it usually presents with a plaque or erythematous papule that progressively increases in size and is ulcerated in the centre.2,3 The visceral form, much more serious, has multiple presentations; it usually begins with unexplained fever which develops over weeks or months, which may or may not be accompanied by haematologic alterations, and progressive multi-organ involvement that may even lead to death.4
According to WHO reports,5–8Leishmania infections are enzootic in almost one hundred countries, including Spain, with a global incidence of 2 million new cases per year. In our country, the disease follows 3 patterns of presentation: (a) endemic, in which cases of disease occur sporadically and dogs act as the main reservoir; (b) cases associated with HIV co-infection or immunosuppressive conditions; and (c) epidemic outbreaks.
In Spain, and therefore also in the Community of Valencia, human leishmaniasis has been monitored since 1982 through the reporting system for Mandatory Reportable Diseases (MRD). In 1995, through Royal Decree 2210, it became notifiable only in the autonomous communities where the disease is endemic. Recently, according to Order SSI/445/2015, it was once again considered a national MRD, with basic epidemiological data to be reported weekly.
In recent years, a significant outbreak of human leishmaniasis, with visceral and cutaneous forms, has been detected. It affects all age groups in municipalities of the southwest of the Community of Madrid and has become notable due to the large number of cases (more than 400 since it began mid-2009) and the discovery of a new reservoir (hares).9,10 In the current study, we present another outbreak that has been detected in the town of Tous (Valencia). Our main objective is to describe the clinical and epidemiological characteristics of the diagnosed cases of leishmaniasis, while mentioning the main Public Health measures established to contain the outbreak.
Material and methodsTous is a municipality in the Community of Valencia, belonging to the province of Valencia, in the region of La Ribera Alta. It is a rural area with a surface area of 127.50km2 and a population of 1314 residents (according to the National Statistics Institute, 2013). The climate is Mediterranean and only 6% of the land is arable, while the rest is populated by vegetation (but not very rich in tree species) or covered by the waters of the Júcar river swamp. Like other areas of the Mediterranean, it is considered an endemic location for canine leishmaniasis.11
From the National Epidemiological Surveillance Network (RENAVE), after the detection of an unusual cluster of cases in Tous in mid-2012, surveillance was intensified with different strategies, including notifying primary care centres of the alert and reinforcing coordination with specialised care. Cases were defined as persons diagnosed with leishmaniasis (ICD-9 085, ICD-10 B55) in compliance with the clinical and laboratory criteria defined by RENAVE,12 who were residents of the town of Tous, and whose date of onset of symptoms was between 1 January 2012 and 31 December 2015 (in 2011, no case was reported in this town).
For the retrospective collection of data, a systematic questionnaire was developed by a multidisciplinary group of medical professionals, containing several variables. The sources of information used were telephone contact with patients, as well as computerised patient records from both the primary care (ABUCASIS system) and specialised care (SIAS system) settings of the Department of Health of La Ribera of the Community of Valencia.
In the present study, we conducted a descriptive analysis of clinicoepidemiological variables, including: age, sex, residence, immunodepression status, risk factors for vectorial transmission, clinical signs and symptoms, diagnosis, treatment and progress. For the purposes of analysis, a complete study of the cases was performed, grouping the cutaneous and visceral forms (the latter was rare).
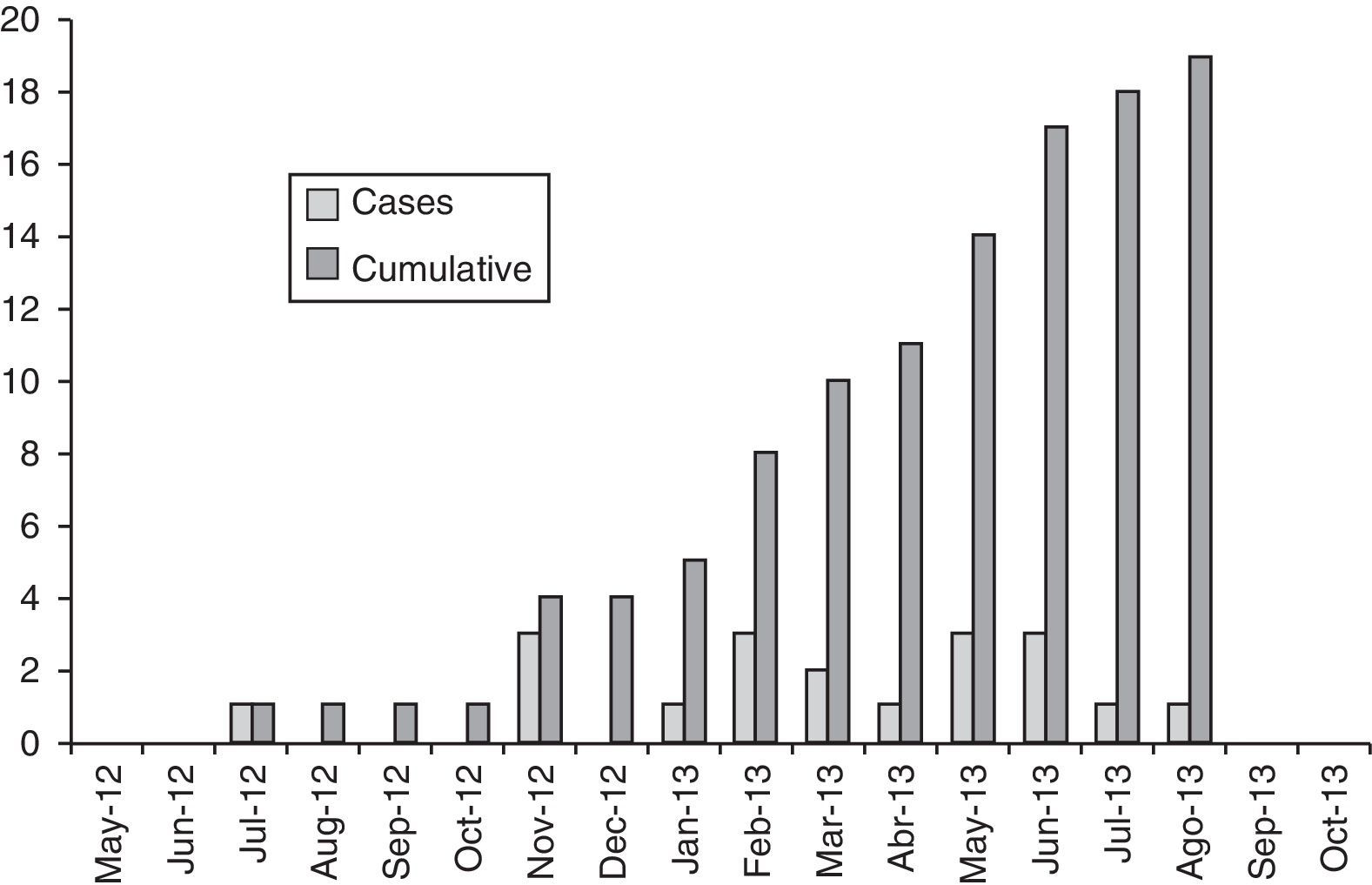
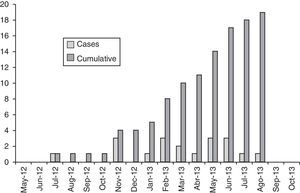
ResultsFrom week 28 of 2012 until week 30 of 2013, a total of 19 patients with leishmaniasis who fulfilled the definition of a case for this outbreak were reported to RENAVE. The calculated incidence rate was 1.44%. Fig. 1 shows the epidemiological curve of the emergence of cases according to the month in which symptoms appeared, and the demographic characteristics and risk factors of reported cases are shown in Table 1.
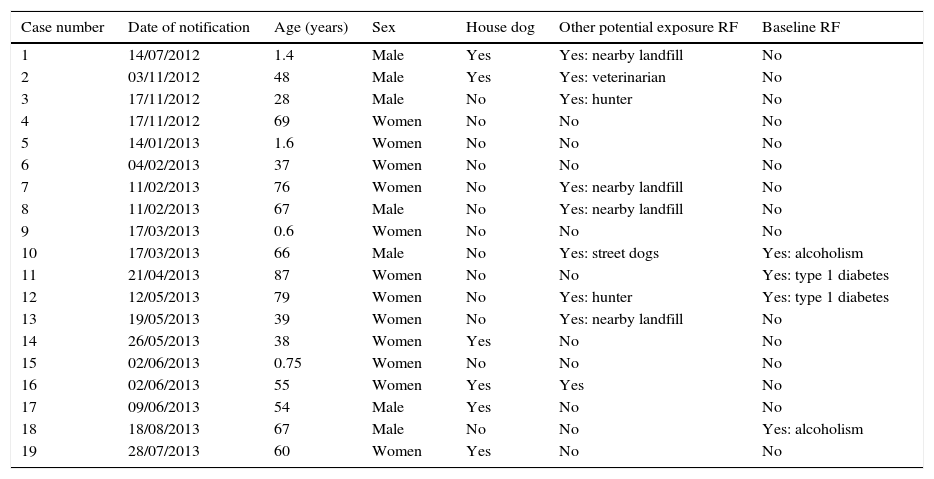
Characteristics of reported cases, including exposure to risk factors (RF) for vector transmission and baseline RF.
| Case number | Date of notification | Age (years) | Sex | House dog | Other potential exposure RF | Baseline RF |
|---|---|---|---|---|---|---|
| 1 | 14/07/2012 | 1.4 | Male | Yes | Yes: nearby landfill | No |
| 2 | 03/11/2012 | 48 | Male | Yes | Yes: veterinarian | No |
| 3 | 17/11/2012 | 28 | Male | No | Yes: hunter | No |
| 4 | 17/11/2012 | 69 | Women | No | No | No |
| 5 | 14/01/2013 | 1.6 | Women | No | No | No |
| 6 | 04/02/2013 | 37 | Women | No | No | No |
| 7 | 11/02/2013 | 76 | Women | No | Yes: nearby landfill | No |
| 8 | 11/02/2013 | 67 | Male | No | Yes: nearby landfill | No |
| 9 | 17/03/2013 | 0.6 | Women | No | No | No |
| 10 | 17/03/2013 | 66 | Male | No | Yes: street dogs | Yes: alcoholism |
| 11 | 21/04/2013 | 87 | Women | No | No | Yes: type 1 diabetes |
| 12 | 12/05/2013 | 79 | Women | No | Yes: hunter | Yes: type 1 diabetes |
| 13 | 19/05/2013 | 39 | Women | No | Yes: nearby landfill | No |
| 14 | 26/05/2013 | 38 | Women | Yes | No | No |
| 15 | 02/06/2013 | 0.75 | Women | No | No | No |
| 16 | 02/06/2013 | 55 | Women | Yes | Yes | No |
| 17 | 09/06/2013 | 54 | Male | Yes | No | No |
| 18 | 18/08/2013 | 67 | Male | No | No | Yes: alcoholism |
| 19 | 28/07/2013 | 60 | Women | Yes | No | No |
The average age of the patients was 46 years (SD 28; range: 0.5–87). Adults were the most affected age group (73%), and 4 cases (27%) occurred in children (all under the age of 2). Nearly two-thirds of patients were women (63%).
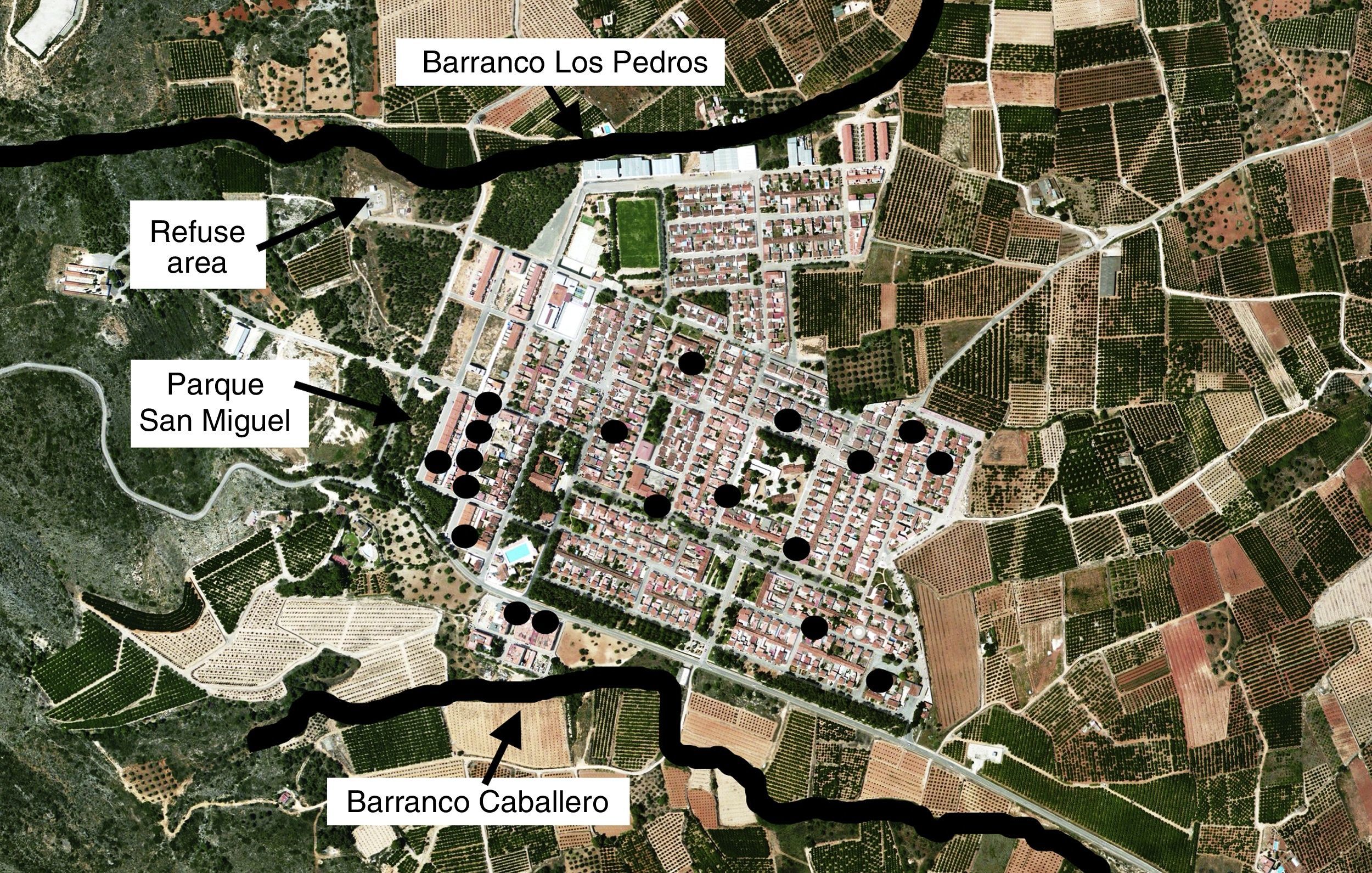
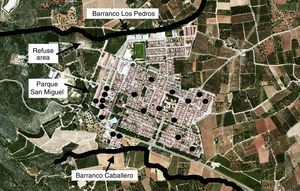
The distribution of the cases covered practically all areas of Tous, although a moderate geographical grouping was noted since 37% of the patients were residents of two neighbouring streets, adjacent to an area with dense vegetation and a waste disposal area (Fig. 2).
58% (11/19) of the patients had some risk factors for vector transmission due to sandfly bites (such as contact with domestic or stray dogs or nearby garbage dumps), and 21% (4/19) presented predisposing immunosuppressive conditions (2 cases of diabetes and 2 of alcoholism); none of the patients had HIV infection.
The most common clinical form was cutaneous leishmaniasis (17/19, 89%). Only 2 cases of visceral leishmaniasis were detected, one of which also had cutaneous manifestations (Table 2). The majority (61%) of patients presented a single cutaneous lesion, while the remaining 49% presented 2 (17%) or 3 (22%) lesions; the mean number of skin lesions per patient was 2 (range: 1–3). The diameter of the lesions was very diverse, ranging from 2 to 20mm (median 9.2mm). The anatomical location of the lesions also varied greatly: 42% on the arms, 32% on the face, 26% on the legs and 11% on the torso. It is worth noting the 2 cases with lesions on the outer ear, since this is a rare location that is difficult to treat and has a tendency for chronification.
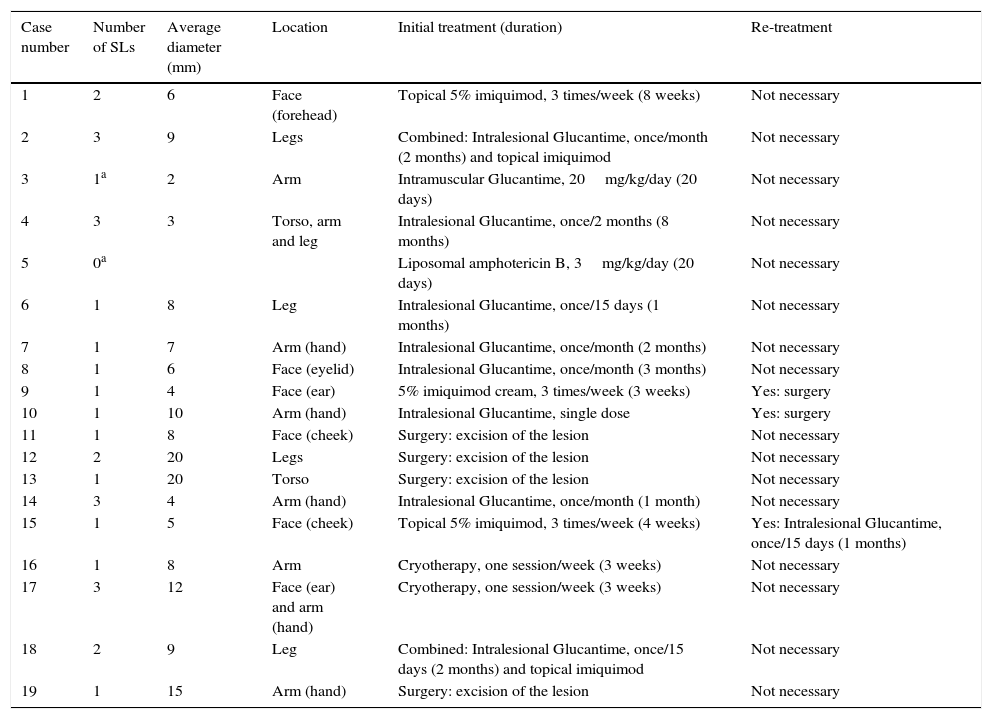
Characteristics of skin lesions (SL) and treatment regimens used.
| Case number | Number of SLs | Average diameter (mm) | Location | Initial treatment (duration) | Re-treatment |
|---|---|---|---|---|---|
| 1 | 2 | 6 | Face (forehead) | Topical 5% imiquimod, 3 times/week (8 weeks) | Not necessary |
| 2 | 3 | 9 | Legs | Combined: Intralesional Glucantime, once/month (2 months) and topical imiquimod | Not necessary |
| 3 | 1a | 2 | Arm | Intramuscular Glucantime, 20mg/kg/day (20 days) | Not necessary |
| 4 | 3 | 3 | Torso, arm and leg | Intralesional Glucantime, once/2 months (8 months) | Not necessary |
| 5 | 0a | Liposomal amphotericin B, 3mg/kg/day (20 days) | Not necessary | ||
| 6 | 1 | 8 | Leg | Intralesional Glucantime, once/15 days (1 months) | Not necessary |
| 7 | 1 | 7 | Arm (hand) | Intralesional Glucantime, once/month (2 months) | Not necessary |
| 8 | 1 | 6 | Face (eyelid) | Intralesional Glucantime, once/month (3 months) | Not necessary |
| 9 | 1 | 4 | Face (ear) | 5% imiquimod cream, 3 times/week (3 weeks) | Yes: surgery |
| 10 | 1 | 10 | Arm (hand) | Intralesional Glucantime, single dose | Yes: surgery |
| 11 | 1 | 8 | Face (cheek) | Surgery: excision of the lesion | Not necessary |
| 12 | 2 | 20 | Legs | Surgery: excision of the lesion | Not necessary |
| 13 | 1 | 20 | Torso | Surgery: excision of the lesion | Not necessary |
| 14 | 3 | 4 | Arm (hand) | Intralesional Glucantime, once/month (1 month) | Not necessary |
| 15 | 1 | 5 | Face (cheek) | Topical 5% imiquimod, 3 times/week (4 weeks) | Yes: Intralesional Glucantime, once/15 days (1 months) |
| 16 | 1 | 8 | Arm | Cryotherapy, one session/week (3 weeks) | Not necessary |
| 17 | 3 | 12 | Face (ear) and arm (hand) | Cryotherapy, one session/week (3 weeks) | Not necessary |
| 18 | 2 | 9 | Leg | Combined: Intralesional Glucantime, once/15 days (2 months) and topical imiquimod | Not necessary |
| 19 | 1 | 15 | Arm (hand) | Surgery: excision of the lesion | Not necessary |
In the clinical diagnosis, all lesions showed similar cutaneous features in the form of plaque or erythematous papules that were greater or smaller size, depending on the time of development; later lesions also presented with central ulcerations. Laboratory diagnoses were based on pathology studies. In all cases, non-necrotising granulomatous chronic dermatitis was observed, with superficial and deep dermal involvement compatible with cutaneous leishmaniasis. Amastigotes were observed in 68% (13/19) of cases, and the presence of DNA was detected by PCR technique in 79% (15/19); in 7 samples were able typified, and the parasite identified as Leishmania infantum. Serology (detection of anti-Leishmania antibodies) was only requested in 4 patients and positive (titre 1/80) in only one of the 2 cases of visceral leishmaniasis.
The analysis of the time transpired from the onset of symptoms to the date of notification showed a median delay of 133 days.
In 31% of cases, physical treatment was considered necessary for the cutaneous lesions (in the form of surgery or cryotherapy), whereas pharmacological treatment was the preferred method in 69% (Table 2). The most commonly used drug was Glucantime (meglumine antimoniate), followed by imiquimod, and 2 patients received combined treatment with both drugs. The evolution was favourable in all cases except in 3 patients (2 of which were initially treated with imiquimod and one with meglumine antimoniate), who were effectively retreated surgically. Some type of residual lesion was observed in 37% of cases after treatment (in the form of a small scar or erythema).
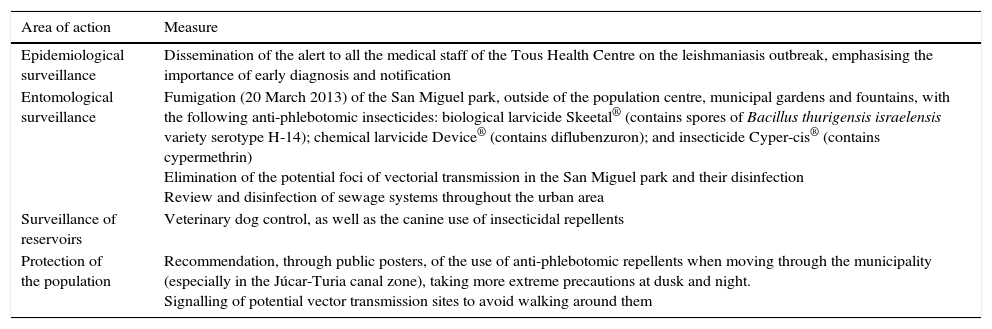
For the prevention and control of the outbreak, a comprehensive action plan was developed, including measures directed at health professionals and control of the vector and reservoir, as well as general recommendations for the population (Table 3).
Main Public Health measures taken in the town of Tous, after the detection of the leishmaniasis outbreak.
| Area of action | Measure |
|---|---|
| Epidemiological surveillance | Dissemination of the alert to all the medical staff of the Tous Health Centre on the leishmaniasis outbreak, emphasising the importance of early diagnosis and notification |
| Entomological surveillance | Fumigation (20 March 2013) of the San Miguel park, outside of the population centre, municipal gardens and fountains, with the following anti-phlebotomic insecticides: biological larvicide Skeetal® (contains spores of Bacillus thurigensis israelensis variety serotype H-14); chemical larvicide Device® (contains diflubenzuron); and insecticide Cyper-cis® (contains cypermethrin) Elimination of the potential foci of vectorial transmission in the San Miguel park and their disinfection Review and disinfection of sewage systems throughout the urban area |
| Surveillance of reservoirs | Veterinary dog control, as well as the canine use of insecticidal repellents |
| Protection of the population | Recommendation, through public posters, of the use of anti-phlebotomic repellents when moving through the municipality (especially in the Júcar-Turia canal zone), taking more extreme precautions at dusk and night. Signalling of potential vector transmission sites to avoid walking around them |
Historically, leishmaniasis in Spain has been geographically associated with the Mediterranean coast; however, the disease has been expanding and now occupies almost the entire national territory (except the Canary Islands). Such is the case that, in recent years, many autonomous communities and cities have registered at least one case of leishmaniasis.13 According to information obtained through the Minimum Basic Data Set (MBDS), in Spain during the period 2000–2010, there were a total of 6220 hospitalisations with a diagnosis of leishmaniasis. In 2739 cases, it was the main diagnosis, which represents a national average rate of 2.8 hospitalisations per 100,000 inhabitants per year.9 It is necessary to keep in mind that unreported cases are quite likely, especially since the cutaneous forms of leishmaniasis are treated mainly on an outpatient basis and therefore do not require hospitalisation. All this only reinforces the fact that the disease is widely distributed and that health professionals must be alert and prepared, regardless of the degree of immunosuppression.14
Although the Mediterranean basin is an endemic area for leishmaniasis, with cases constantly being reported, no outbreaks have been published in the Community of Valencia, and this present report is the first group of cases documented in our area. In the Community of Valencia, the zoonosis report for 2012 shows the notification of 57 cases of leishmaniasis (35 in Valencia, 17 in Alicante, 5 in Castellón), 86% of which were confirmed.15 The highest rate was in the province of Valencia with 1.36 cases/100,000 residents, followed by Alicante with 0.88 cases/100,000 residents, and Castellón with 0.83 cases/100,000 residents. According to this report, there has been a progressive increase in the last 3 years; the highest rate in the last decade was recorded in 2012, and the city of Valencia was the urban area with the highest number of cases registered in 2012 (with a total of 10 cases).
At the beginning of 2013, the Public Health Administration initiated a series of actions in the town of Tous in order to control and prevent the emergence of new cases. The main measures introduced have been summarised in Table 3. The last reported case was in week 30 of 2013, and subsequently only 2 isolated cases of cutaneous leishmaniasis have been detected (one in the week 15 and the other in week 51 of 2015), so we believe that measures implemented by the Public Health Administration have been effective.
One of the most effective measures for controlling leishmaniasis is early diagnosis and effective management of cases through epidemiological surveillance of the disease. However, this is sometimes difficult due to the prolonged incubation period (weeks, months, or even years). Thus, strengthened prevention systems, effective exchange of information between medical staff and the Public Health Administration, and the rapid dissemination of medical alerts, as well as surveillance protocols and disease management among professionals in the area, are critical to avoid delays in diagnosis and reporting. One study has confirmed that the active search for cases through clinical signs and serological studies involves a significantly shorter delay in the treatment of patients with leishmaniasis (27 days vs 47 days) than the passive search of cases through registration of newly diagnosed patients awaiting treatment. The authors conclude that active case-finding significantly increases disease incidence data, and that the measure is also cost-effective.16
The main risk factor associated with the increase in the incidence of leishmaniasis is the presence of sandflies (phlebotomus spp.) infected with L. infantum. Therefore, the control of the vectors is a key factor in stopping and reducing the transmission of the disease, especially in the domestic and peridomestic context.9 The knowledge of the seasonal circulation of the adult sandfly and the identification of the species involved are crucial for monitoring and control. In Spain, Phlebotomus perniciosus and Phlebotomus ariasi have been shown to be the main vectors involved in parasite transmission, and to a lesser extent Phlebotomus langeroni and Phlebotomus longicuspis, with their period of activity being from May to October.17P. perniciosus is the most abundant and is widely distributed throughout most of the arid zones of the peninsula and the Balearic archipelago, while P. ariasi is linked to cooler and more humid environments of the peninsula.18
The use of barrier methods (both chemical and physical) to prevent bites, environmental management to prevent the proliferation of insects (such as avoiding stagnant water and the elimination of waste dumps and surrounding breeding sites), improved housing conditions, and personal protection systems are the main methods described for combating arthropod vectors. One study has detected an overall reduction of 59% (95% CI: 25–78%) in cases of leishmaniasis 17–20 months after the introduction of fine mesh, insecticide-infused mosquito screens in 155 villages affected by the disease.19 Another study has shown that the cumulative incidence and relative risk (RR) of cutaneous leishmaniasis decreased (from RR=1.14 [CI 95%: 1.05–1.23] in 2007 to RR=0.84 [95% CI: 0.72–0.98] in 2010) 4 months after having impregnated screens and curtains with the insecticide deltamethrin.20 The use of aerosol insecticides is one of the main methods for vector control, although the sensitivity spectrum of sandflies to different insecticides is not fully understood. Resistance to organochlorines, malathion and pyrethroids is low, but there have been reports, so it is recommended to use different insecticides at appropriate intervals.5 Unlike mosquitos, sandflies do not have an aquatic larval phase. In most species the breeding sites are unknown, so vector control through the larval reservoir is ineffective.
Control of animal reservoirs is complex and needs to be adapted to the local situation. In general, exhaustive veterinary control of animals (especially the canine population) and identification, where appropriate, of the main reservoir are 2 key factors in the prevention of leishmaniasis. In our outbreak, epidemiological surveys of patients reported contact with dogs (mostly), partridges and cats. Classically, dogs are considered the main reservoir of leishmaniasis, so the likelihood that they are also the reservoir in this outbreak is high; however, a more comprehensive study is required to confirm that dogs have been the reservoir in our study. In Spain, 5–10% of the canine population is infected by Leishmania,11 so determining the proportion of infected dogs in a geographical area, or those which are infected over a period of time, may be a sentinel indicator of the prevalence of infection.21
Finally, the protection of the population is another of the transcendental measures to reduce the incidence of the disease. The diffusion of individual protection measures and the zones of greater risk of transmission, as well as the measures for reducing contact between the host and sandflies, are factors to consider. Several studies have shown that the use of collars containing deltamethrin and permethrin pipettes (with or without imidacloprid), as well as prophylactic use with domperidone, significantly reduces the proportion of infected dogs.22 In addition, several vaccines have been marketed in recent years (such as Leishmune® and CaniLeish®) which appear to significantly reduce the proportion of parasite-infected dogs, although further randomised clinical trials are required to clearly establish vaccine efficacy as a leishmaniasis control measure.23
Among the limitations of our study is the lack of antigenic and genomic characterisation of the Leishmania strains detected, in order to establish epidemiological links with other future outbreaks. Likewise, there have been no field studies in vector insects and potential reservoirs (dogs) in order to identify the possible focus and thereby estimate whether the control measures were effective.
The transmission of leishmaniasis is a complex biological system that involves the human host, parasite, vector and reservoir animal.
Control strategies must be comprehensive, multifactorial and adapted to each situation. After our experience, we believe essential measures include the rapid diffusion of epidemiological alerts, introduction of environmental measures to prevent the proliferation of sandflies, identification of the main reservoir, and personal protection systems.
FundingNo funding was received for the completion of this article.
Conflicts of interestNone of the authors has a conflict of interest with the study submitted.
Please cite this article as: Roth-Damas P, Sempere-Manuel M, Mialaret-Lahiguera A, Fernández-García C, Gil-Tomás JJ, Colomina-Rodríguez J, et al. Brote comunitario de leishmaniasis cutánea en la comarca de La Ribera: a propósito de las medidas de Salud Pública. Enferm Infecc Microbiol Clin. 2017;35:338–343.