In 2011, a hospital-wide outbreak of OXA-48 producing Klebsiella pneumoniae occurred in our hospital, an epidemiological setting of high ESBL-producing K. pneumoniae rates. This study identifies risk factors for colonization with carbapenemase-producing enterobacteria (CPE) at Surgical Intensive Care Unit (SICU) admission.
MethodsA 2-year retrospective study was performed in all patients admitted to the SICU that following routine had a rectal swab collected upon admission.
ResultsOf 254 patients admitted, 41 (16.1%) harbored CPE (five showing two carbapenemase-producing isolates). Most frequent carbapenemase-producing isolates and carbapenemases were K. pneumoniae (39/46, 84.8%) and OXA-48 (31/46; 76.1%), respectively. Carriers significantly had higher rates of chronic renal disease, previous digestive/biliary endoscopy, hospitalization, ICU/SICU admission, intraabdominal surgery, and antibiotic intake, as well as higher median values of clinical scores (SOFA, SAPS II and APACHE II). In the multivariate analysis (R2=0.309, p<0.001), CPE carriage was associated with prior administration of 3rd–4th generation cephalosporins (OR=27.96, 95%CI=6.88, 113.58, p<0.001), β-lactam/β-lactamase inhibitor (OR=11.71, 95%CI=4.51, 30.43, p<0.001), abdominal surgery (OR=6.33, 95%CI=2.12, 18.89, p=0.001), and prior digestive/biliary endoscopy (OR=3.88, 95%CI=1.56, 9.67, p=0.004).
ConclusionsA strong association between production of ESBLs and carriage of CPE (mainly OXA-48 producing K. pneumoniae) was found. According to the model, the co-selection of β-lactamases by previous exposure to broad-spectrum cephalosporins and β-lactam/β-lactamase inhibitors (with lower relative risk), abdominal surgery and prior digestive/biliary endoscopy were factors associated with CPE carriage.
En 2011 se produjo un brote epidémico de Klebsiella pneumoniae productor de OXA-48 en nuestro hospital, un entorno epidemiológico de altas tasas de K. pneumoniae productor de BLEE. Este estudio identifica factores de riesgo de colonización por enterobacterias productoras de carbapenemasas (EPC) en el momento del ingreso en la unidad de cuidados críticos quirúrgicos (UCCQ).
MétodosSe realizó un estudio retrospectivo durante 2 años en todos los pacientes ingresados en la UCCQ a los que, siguiendo la rutina habitual, se les tomaba un hisopo rectal en el momento de ingreso.
ResultadosDe los 254 pacientes ingresados, 41 (16,1%) portaban EPC (5 con 2 aislados productores de carbapenemasas). Los aislados productores de carbapenemasas y las carbapenemasas más frecuentes fueron K. pneumoniae (39/46, 84,8%) y OXA-48 (31/46; 76,1%), respectivamente. Los portadores presentaban de forma significativa mayor frecuencia de insuficiencia renal crónica, historia previa de endoscopia digestiva/biliar, hospitalización, ingreso previo en UCI/UCCQ, cirugía intraabdominal y exposición a antibióticos, así como valores más altos (mediana) de SOFA, SAPS II y APACHEII. En el análisis multivariado (R2=0,309; p<0,001), el estado de portador de EPC se asoció con la administración previa de cefalosporinas de amplio espectro (OR=27,96; IC95%: 6,88-113,58; p<0,001), β-lactámicos/inhibidores de β-lactamasas (OR=11,71; IC95%: 4,51-30,43; p<0,001), cirugía abdominal (OR=6,33; IC95%: 2,12-18,89; p=0,001) y endoscopia digestiva/biliar previa (OR=3,88; IC95%: 1,56-9,67; p=0,004).
ConclusionesSe encontró una fuerte asociación entre la producción de BLEE y la portación de EPC (fundamentalmente K. pneumoniae productora de OXA-48). De acuerdo con el modelo, la co-selección de β-lactamasas tras exposición previa a cefalosporinas de amplio espectro y en menor medida a β-lactámicos/inhibidores de β-lactamasas, la cirugía abdominal y la endoscopia digestiva/biliar previa fueron factores asociados a la portación de EPC.
In recent years, Enterobacteriaceae have increased their potential for pan-resistance by acquiring resistance to carbapenems through mutations in penicillin binding proteins (uncommon in gram-negatives), reduced permeability in combination with β-lactamases as extended-spectrum β-lactamase (ESBLs), upregulation of efflux systems along with hyperproduction of AmpC β-lactamases, and/or mainly through production of carbapenemases. Klebsiella pneumoniae has the potential to become extensively drug resistant through production of carbapenemases1 as KPC (class A), VIM (class B) and OXA (class D). OXA-48 is by far the most common carbapenemase type circulating in Spain, and is mainly produced by K. pneumoniae1. This enzyme hydrolyses penicillins and carbapenems (although conferring low level resistance to carbapenems),2 and has very weak activity against expanded-spectrum cephalosporins.3 Clonally related OXA-48 producing strains usually co-produce CTX-M-15 β-lactamase2,4,5 and harbor resistance traits to quinolones and aminoglycosides.2,6
Hospital readmission, stay in the Intensive Care Unit (ICU), invasive procedures and prior exposure to broad-spectrum antibiotics (carbapenems, cephalosporins, quinolones) have been identified as risk factors for colonization with carbapenem-resistant Enterobacteriaceae (CPE).7–9 Risk factors for enteric colonization by KPC-producing K. pneumoniae upon ICU admission were prospectively determined in a published study showing association of colonization with prior ICU stay, chronic obstructive pulmonary disease as underlying condition, and previous use of carbapenems and β-lactam/β-lactamase inhibitors.10 Once colonized, long-term carriage of KPC-producing K. pneumoniae has been reported, with multiple hospitalizations and carbapenem-resistant K. pneumoniae infections extending the duration of carriage.11 Subsequent development of infection by carbapenem-resistant K. pneumoniae among colonized patients reached 46% for ICU patients in a previous study,12 with invasive procedures, antipseudomonal penicillins or carbapenem therapy and mechanical ventilation, common factors in patients admitted to ICUs, as risk factors for infection.7
Knowledge of colonization with CPE is important to prevent nosocomial spread and to empirically initiate appropriate therapy in case of infection. Nevertheless, studies exploring risk factors for colonization with CPE did not include a large enough number of OXA-48 producing bacteria, probably due to the recent abrupt emergence of this carbapenemase. In 2011, a hospital-wide outbreak of OXA-48 producing K. pneumoniae occurred in our hospital, an epidemiological setting of high rates of ESBL-producing K. pneumoniae and sustained low level of VIM-producing bacteria.2 In this context, the aim of the present study was to identify risk factors for colonization with CPE upon Surgical ICU (SICU) admission in our hospital in the two-year period after the reported outbreak.
MethodsA retrospective, observational study was carried out from January 2012 to December 2013 analyzing data from all patients >14 years of age admitted to the SICU of Hospital Universitario La Paz (Madrid, Spain), a general reference hospital with ≈1300 beds and ≈50,000 admissions/year, and a SICU of 15 beds with a median occupancy rate of 80%-90%. All patients admitted to the SICU have a rectal swab collected at entry in the Unit as part of the management routine. The study protocol was approved by the Ethical Review Board of Hospital La Paz.
Clinical records were reviewed to obtain demographic (age, gender, nursing home residency…) and clinical (underlying conditions) data, and the Sequential Organ Failure Assessment (SOFA),13 the Simplified Acute Physiology Score (SAPS II),14 and the Acute Physiologic and Chronic Health Evaluation (APACHE) II15 scores were calculated with data at SICU admission. Antibiotic intake for >3 days in the previous six months, hospitalizations in the last 12 months, intraabdominal and/or urgent surgery in the previous 30 days, and ward and number of days of hospitalization prior to SICU admission were recorded. Microbiological data from rectal swabs collected within the first 24h of SICU admission following our screening routine to all patients entering the SICU were recorded. Carriers were defined as patients without signs/symptoms of infection that had CPE isolated from the rectal swab collected at SICU entry, and non-carriers as patients without signs/symptoms of infection and no CPE isolation.
Microbiological methodsRectal swabs collected were immediately sent to the Microbiology department for culture on McConkey agar plates containing 4mg/L of cefotaxime. Identification was performed using matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF; Bruker Daltonics, Inc., Billerica, MA) system. ESBLs and carbapenemases were phenotypically confirmed with the double-disk synergy method with cefotaxime, ceftazidime, cefotaxime/clavulanate and ceftazidime/clavulanate and the modified Hodge test using imipenem, meropenem and ertapenem discs. PCRs with specific primers were used for the detection of blaKPC, blaVIM, blaIMP, blaNDM and blaOXA-48 genes16–18 and other β-lactamase genes (TEM, SHV, CTX-M, and OXA-1).19
The genetic relationships between the OXA-48-producing K. pneumoniae isolates were determined by MLST according to the Institute Pasteur Scheme.20 Clone-specific real time PCR for K. pneumoniae ST11 and ST405 was also used for rapid typing.21
Statistical analysisComparison of proportions was performed by chi-square test and Fisher's exact test when necessary. For quantitative variables, since data did not show normality in the Kolmogorov–Smirnoff test, the Kruskal–Wallis and Mann–Whitney tests were used when necessary. Bivariate analyses were performed to compare all variables between carriers and non-carriers. Logistic regression models (stepwise procedure) were performed using “carriage of CPE” as dependent variable and those showing differences (p<0.05) in bivariate analyses as independent variables. Interactions and linear dependence between independent variables were previously controlled. The statistical analysis was performed using SPSS, version 4 (SPSS Inc., Chicago, IL). The model showing the maximum parsimony (the lowest number of variables with no significant reduction in the value of the determination coefficient) and the highest R2 was considered.
ResultsA total of 254 patients (65.5±15.5 years; 63.4% males) were admitted to the SICU during the study period, 41 (16.1%) of them harboring CPE (five showing co-existence of two carbapenemase-producing isolates) and 45 (17.7%) ESBL-producing isolates. A strong association between carriage of ESBL and CPE was found (χ2=153.49, p<0.0001); the percentage of patients harboring ESBL-producing bacteria being significantly higher among carbapenemase carriers (85.4% vs. 4.7%, p<0.001).
A total of 46 carbapenemase-producing isolates were found, most of them were K. pneumoniae (39 out of 46; 84.8%). OXA-48 was the most frequent carbapenemase (31 out of 46; 76.1%). Twenty-two out of these 31 (71.0%) OXA-48 K. pneumoniae could be genome sequenced. Of them, 13 (59.1%) were ST11, 7 (31.8%) were ST405, and 2 (9.1%) were ST323. Table 1 details bacterial species and carbapenemases found in carriers. The profile of β-lactamase genes was different in ST11 (TEM-116, SHV-11 and OXA-1) and ST405 (TEM-1, SHV-76, CTX-M-15 and OXA-1) OXA-48-producing K. pneumoniae.
Species, carbapenemases and ESBLs found in the 41 carbapenemase carriers.
| No. of patients | OXA-48 | VIM-1 | KPC | No. patients co-presenting ESBL |
|---|---|---|---|---|
| 28 | K. pneumoniae | 27 | ||
| 2 | E. coli | 1 | ||
| 1 | K. pneumoniae+E. coli | 1 | ||
| 4 | K. pneumoniae | 2 | ||
| 1 | K. oxytoca | 0 | ||
| 1 | K. pneumoniae+E. coli | 1 | ||
| 1 | K. pneumoniae | K. pneumoniae | 0 | |
| 1 | K. oxytoca | K. pneumoniae | 1 | |
| 1 | K. pneumoniae | P. aeruginosa | 1 | |
| 1 | K. pneumoniae | 1 |
Table 2 shows demographic data, comorbidities and severity (clinical scores) of the study patients distributed by CPE carriage (carriers/non-carriers). Only four patients lived in a nursing home, and only one among carriers. The percentage of patients presenting respiratory insufficiency at SICU admission or chronic renal disease was significantly higher for CPE carriers as occurred with median values of clinical scores (SOFA, SAPS II and APACHE II).
Demographic data, comorbidities and severity (clinical scores); data expressed as n (%) except where indicated.
| Total (n=254) | Carriers (n=41) | Non-carriers (n=213) | p | |
|---|---|---|---|---|
| Age, mean±SD | 65.5±15.5 | 66.9±17.5 | 65.2±15.1 | 0.519 |
| Males | 161 (63.4) | 25 (61.0) | 136 (63.8) | 0.726 |
| Diabetes mellitus | 57 (22.4) | 11 (26.8) | 46 (21.6) | 0.462 |
| Malignancies | 57 (22.4) | 12 (29.3) | 45 (21.1) | 0.253 |
| COPD | 41 (16.1) | 7 (17.1) | 34 (16.0) | 0.859 |
| Respiratory insufficiency at SICU admission | 30 (11.9) | 10 (24.4) | 20 (9.4) | 0.007 |
| Congestive heart disease | 17 (6.7) | 3 (7.3) | 14 (6.6) | 0.743 |
| Steroids intake | 10 (3.9) | 4 (9.8) | 6 (2.8) | 0.059 |
| Chronic renal disease (dialysis) | 9 (3.5) | 4 (9.8) | 5 (2.3) | 0.040 |
| Cirrhosis | 7 (2.8) | 3 (7.3) | 4 (1.9) | 0.086 |
| SOFA at SICU admission, median (P25–P75) | 3.0 (2.0–6.0) | 6.0 (3.0–8.0) | 3.0 (1.0–5.0) | <0.001 |
| SAPS II at SICU admission, median (P25–P75) | 30.0 (25.0–42.0) | 40.0 (30.5–52.5) | 30.0 (25.0–39.5) | 0.002 |
| APACHE II at SICU admission, median (P25–P75) | 12.0 (8.0–16.0) | 15.0 (11.0–22.5) | 11.0 (8.0–15.0) | <0.001 |
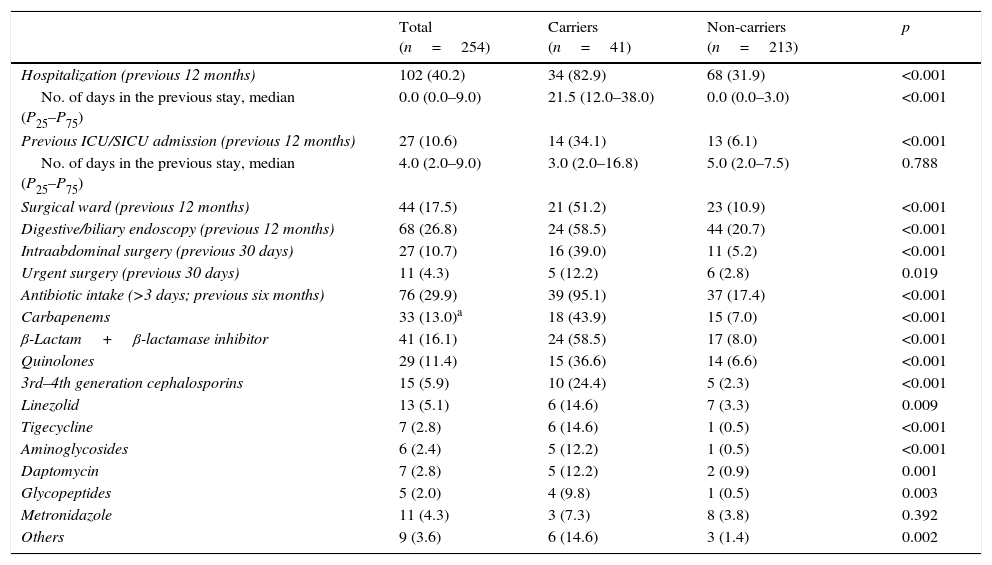
Table 3 shows data on hospitalization in the previous 12 months, surgical events in the preceding month and antibiotic intake the last six months for carriers and non-carriers. Hospitalizations, prior ICU/SICU admission and digestive/biliary endoscopy in the last 12 months, as well as intraabdominal surgery in the previous 30 days were significantly more frequent in carriers. Overall antibiotic intake in the last six months was significantly more common in carriers, with more frequent administration of all antibiotic compounds/classes (except metronidazole) than in non-carriers.
Hospitalizations, surgical events and antibiotic intake; data expressed as n (%) except where indicated.
| Total (n=254) | Carriers (n=41) | Non-carriers (n=213) | p | |
|---|---|---|---|---|
| Hospitalization (previous 12 months) | 102 (40.2) | 34 (82.9) | 68 (31.9) | <0.001 |
| No. of days in the previous stay, median (P25–P75) | 0.0 (0.0–9.0) | 21.5 (12.0–38.0) | 0.0 (0.0–3.0) | <0.001 |
| Previous ICU/SICU admission (previous 12 months) | 27 (10.6) | 14 (34.1) | 13 (6.1) | <0.001 |
| No. of days in the previous stay, median (P25–P75) | 4.0 (2.0–9.0) | 3.0 (2.0–16.8) | 5.0 (2.0–7.5) | 0.788 |
| Surgical ward (previous 12 months) | 44 (17.5) | 21 (51.2) | 23 (10.9) | <0.001 |
| Digestive/biliary endoscopy (previous 12 months) | 68 (26.8) | 24 (58.5) | 44 (20.7) | <0.001 |
| Intraabdominal surgery (previous 30 days) | 27 (10.7) | 16 (39.0) | 11 (5.2) | <0.001 |
| Urgent surgery (previous 30 days) | 11 (4.3) | 5 (12.2) | 6 (2.8) | 0.019 |
| Antibiotic intake (>3 days; previous six months) | 76 (29.9) | 39 (95.1) | 37 (17.4) | <0.001 |
| Carbapenems | 33 (13.0)a | 18 (43.9) | 15 (7.0) | <0.001 |
| β-Lactam+β-lactamase inhibitor | 41 (16.1) | 24 (58.5) | 17 (8.0) | <0.001 |
| Quinolones | 29 (11.4) | 15 (36.6) | 14 (6.6) | <0.001 |
| 3rd–4th generation cephalosporins | 15 (5.9) | 10 (24.4) | 5 (2.3) | <0.001 |
| Linezolid | 13 (5.1) | 6 (14.6) | 7 (3.3) | 0.009 |
| Tigecycline | 7 (2.8) | 6 (14.6) | 1 (0.5) | <0.001 |
| Aminoglycosides | 6 (2.4) | 5 (12.2) | 1 (0.5) | <0.001 |
| Daptomycin | 7 (2.8) | 5 (12.2) | 2 (0.9) | 0.001 |
| Glycopeptides | 5 (2.0) | 4 (9.8) | 1 (0.5) | 0.003 |
| Metronidazole | 11 (4.3) | 3 (7.3) | 8 (3.8) | 0.392 |
| Others | 9 (3.6) | 6 (14.6) | 3 (1.4) | 0.002 |
In the multivariate analysis (R2=0.309, p<0.001), carriage of CPE was associated with prior administration of 3rd–4th generation cephalosporins (OR=27.96, 95%CI=6.88, 113.58, p<0.001), β-lactam/β-lactamase inhibitor (OR=11.71, 95%CI=4.51, 30.43, p<0.001), abdominal surgery (OR=6.33, 95%CI=2.12, 18.89, p=0.001), and prior digestive/biliary endoscopy (OR=3.88, 95%CI=1.56, 9.67, p=0.004).
Sixteen out of the 41 (39.0%) carriers developed infection, and the same CPE isolate found in the rectal swab at SICU admission was isolated from blood (4 samples), urine (5 samples), bronchoalveolar lavage/tracheal aspiration (6 samples), and intra-abdominal samples (7 samples).
DiscussionThe present study exploring risk factors for asymptomatic carriage of CPE at SICU admission showed a strong correlation between carriage of ESBLs and carbapenemases. This strong correlation is in accordance with the results of a prospective study in our country describing high rates of coproduction in K. pneumoniae.22 Main factors associated with CPE carriage were prior administration of broad-spectrum cephalosporins and β-lactam/β-lactamase inhibitor, and prior invasive procedures as abdominal surgery and digestive/biliary endoscopy. Several recent studies on CPE, mainly K. pneumoniae, showed that ICU stay, poor functional status and nursing home residency were predictors of asymptomatic colonization by carbapenemase-resistant K. pneumoniae.7,23,24 The results of our bivariate analysis were in accordance with this since ICU stay was significantly more frequent and mean values of clinical scores were significantly higher among carriers. The percentage of patients living in a nursing home was surprisingly low and thus, no conclusions can be drawn. Other factors described in the literature are exposure to invasive procedures and/or to antimicrobials.7,25 These, among all factors identified in our bivariate analysis, were the type of factors that remained significantly associated with CPE carriage in the multivariate analysis performed. In relation to this, the association between biliary endoscopy and biliary carriage of resistant bacteria had been previously described by our group.26 Once colonized, invasive procedures were also identified as risk factor for infection by CPE in published studies.24,27
In relation to antibiotic exposure, genes encoding carbapenemases are commonly found in mobile genetic elements, allowing the organism to acquire genes conferring resistance to other antibiotic classes, such as other β-lactamases, aminoglycoside-modifying enzymes and determinants for fluoroquinolone resistance.28 Due to this, CPE are increasingly multidrug resistant; this allowing co-selection of resistance by exposure to other antimicrobial groups. In the present study, antibiotic exposure in the previous six months was significantly more common among carriers. Previous overall antibiotic exposure23,24 and specifically use of carbapenems (described as risk factor also for infection)24 and cephalosporins29 have been reported as risk factors for carbapenem-resistant K. pneumoniae colonization. In addition, exposure to fluoroquinolones has also been identified as an independently predictive factor for carriage.23
OXA-48 hydrolyzes penicillins (with or without inhibitor) and carbapenems (low level of resistance), but not 3rd generation cephalosporins.30 OXA-48 producing isolates have been reported, according to CLSI, as carbapenem-susceptible.31 However, many OXA-48-producing Enterobacteriaceae coproduce an ESBL, jeopardizing all regular beta-lactam antibiotics.32 In the present study, where the majority of patients were colonized with OXA-48 producing Klebsiella spp., only prior administration of 3rd–4th generation cephalosporins and β-lactam/β-lactamase inhibitor (with lower OR) were associated with colonization in the multivariate analysis. Most OXA-48 producing K. pneumoniae strains co-produce CTX-M-15,4,5 co-selection of both enzymes may be enhanced by prior exposure to 3rd–4th generation cephalosporins. According to data in the present study, this co-selection seemed to be phenotypical and not clonal-related since there was a polyclonal pattern (with a predominant clone, but with two different clones accounting for approximately 40% cases) among the 22 isolates that could be sequenced.
Despite the screening method used in the present study detected carbapenemases regardless the carbapenem MIC for the isolates, one study limitation could be the reported inappropriateness of the method for CPE in an environment where OXA-48 is the predominant carbapenemase33 due to the inhibition of OXA-48 isolates by the cefotaxime concentration used. However, in a previous study in our hospital including all OXA-48 isolates from December 2010 to August 2013, only 8% OXA-48 isolates (24 out of 300 patients with OXA-48 isolates) were cefotaxime-susceptible.34 Therefore, although we cannot dismiss the possibility of not having detected possible cefotaxime-susceptible OXA-48 isolates among non-carriers, the weight of it in the group of 213 non-carriers would not be significant facing published data on susceptibility in our hospital.
Since the first description of OXA-48 in 2009 in Spain,4 reports of isolates to the Spanish Centre for Microbiology have increased from zero cases in 2010 to 163 cases in 2012.1 The association of production of ESBLs and OXA-48, allows selection by antibiotics target of these enzymes as broad-spectrum cephalosporins. This, and the possible association with resistance to other antibiotic groups, is a worrisome problem that should be monitored, more even when resistance to carbapenems can emerge by the acquisition of β-lactamase genes in isolates with permeability alterations (efflux pumps and/or porine deficit).
Monitoring of carriage acquires importance since colonized patients could develop infection by colonizing bacteria. In the present study up to 39% patients developed infection by the CPE isolated from rectal swabs.
This study, to our knowledge the first study exploring risk factors for carriage of CPE at SICU admission, identified a strong association between production of ESBLs and carriage of CPE (mainly OXA-48 producing K. pneumoniae). The co-selection of β-lactamases by previous exposure to broad-spectrum cephalosporins and β-lactam/β-lactamase inhibitors (with lower relative risk), abdominal surgery and prior digestive/biliary endoscopy were factors associated with CPE carriage. These facts are important in epidemiological settings with high rates of ESBL-producing K. pneumoniae as our hospital, and in ICUs that are commonly ESBL endemic environments. The high rate of colonized patients developing infection in the present study warrants further studies to determine risk factors for infection in carriers of this type of CPE.
FundingData entry and analysis of this study was supported in part by an unrestricted grant from Fundación Biomédica del Hospital Universitario La Paz (Madrid, Spain). The funding body had no decision in design, collection, analysis, interpretation of data, manuscript preparation or decision about journal to be submitted.
Conflicts of interestEM has received payments for lectures from Astellas Pharma, Pfizer and Merck Sharp & Dohme. M-JG and LA are employees of PRISM-AG that has received an investigational grant for data analysis. All other authors declare no conflicts of interest.