Tuberculous meningitis (TBM), the most serious form of tuberculosis, results in high mortality and long-term disability in low-resource countries. We investigated temporal trends in mortality and sequelae in a high-resource low-incidence country.
MethodsWe performed a retrospective cohort study of all adult patients with TBM at two third-level teaching hospitals in Barcelona (Spain), between January 1990 and December 2017, assessing temporal trends in mortality and sequelae after 12 months over four consecutive 7-year time windows. Rates observed across the four periods were adjusted for covariates.
ResultsOf the 135 cases included, all but one started tuberculosis (TB) treatment and 120 (89.6%) received rifampicin, isoniazid, and pyrazinamide, with or without ethambutol. The probability of being alive at month 12 was 81.8%, with no differences among the four periods: in comparison with the 1990–1996 period, the adjusted hazard ratios and 95% confidence intervals (CI) were 2.55 (0.71–9.25), 0.70 (0.13–3.85), and 1.29 (0.28–5.91) for the 1997–2003, 2004–2010, and 2011–2017 periods respectively. Sequelae were present in 28.3% at month 12, with no differences across the four periods in the adjusted analysis: in comparison with the 1990–1996 period, the odds ratios and 95% CIs were 0.80 (0.09–7.22); 1.94 (0.21–17.96), and 2.42 (0.25–23.07) for the 1997–2003, 2004–2010, and 2011–2017 periods respectively.
ConclusionThis study shows that TBM still causes high mortality and disability even in a high-resource low-incidence TB setting and without improvement over time.
La meningitis tuberculosa (TBM), la forma más grave de tuberculosis, provoca una alta mortalidad y discapacidad a largo plazo en países con bajos recursos. Nuestro objetivo es investigar la tendencia temporal de la mortalidad y las secuelas en un país con recursos elevados y baja incidencia.
MétodosHemos realizado un estudio de cohortes retrospectivo de los pacientes adultos con TBM en dos hospitales universitarios de tercer nivel en Barcelona (España), entre 1990 y 2017, evaluando las tendencias temporales de mortalidad y secuelas a los 12 meses, comparando cuatro periodos consecutivos de siete años. Las tasas observadas en los cuatro periodos se han ajustado por covariables.
ResultadosDe los 135 casos incluidos, todos menos uno inició tratamiento antituberculoso y 120 (89,6%) recibieron rifampicina, isoniazida y pirazinamida, con o sin etambutol. La probabilidad de estar vivo a los 12 meses fue de 81,8%, sin diferencias entre los cuatro periodos: en comparación con el periodo 1990-1996, los coeficientes de riesgo ajustados y los intervalos de confianza (IC) del 95% fueron 2,55 (0,71-9,25), 0,70 (0,13-3,85) y 1,29 (0,28-5,91) para los periodos 1997-2003, 2004-2010 y 2011-2017, respectivamente. Las secuelas estaban presentes en 28,3% en el mes 12, sin diferencias entre los cuatro periodos en el análisis ajustado: en comparación con el periodo 1990-1996, los coeficientes de probabilidad y los IC 95% fueron 0,80 (0,09-7,22); 1,94 (0,21-17,96) y 2,42 (0,25-23,07) para los periodos 1997-2003, 2004-2010 y 2011-2017, respectivamente.
ConclusiónEste estudio muestra que la TBM todavía causa una alta mortalidad y discapacidad sin mejoría con el tiempo, incluso en un entorno con baja incidencia de tuberculosis y con elevados recursos.
Tuberculous meningitis (TBM) is the most serious form of tuberculosis (TB), carrying high rates of both morbidity and mortality.1 Despite improvements in neuroimaging,2 diagnostic tests,3 the optimization of antimicrobial therapy,4,5 and the systematic use of adjuvant corticosteroids,6,7 long-term disabilities and mortality remain stubbornly high.8 A systematic review and meta-analysis, including more than 5000 patients, reported 6-month mortality rates of 5% to 67% in Asian studies and 23–80% in African studies, with no significant changes observed over time.9 However, these high death rates may reflect the negative effects of HIV infection and under-equipped healthcare systems in those regions, precluding extrapolation to high-resource and low-incidence TB settings. Few data exist for high-resource countries,10 and even less in those with universal access to healthcare.
In this study, we aimed to report the prognosis of TBM and how this has evolved over time in two large teaching hospitals in a country with a low incidence of TB and universal healthcare.
MethodsDesign and study populationWe conducted a retrospective observational study of patients with TBM, aged 18 or older, treated at two tertiary teaching hospitals in Spain between January 1990 and December 2017. The patient's clinical records were reviewed to confirm the presumed diagnosis and to collect epidemiological, clinical, microbiological, treatment, and outcome data. Cases were classified according to previously published diagnostic certainty criteria, as definite, probable, or possible11 (Supplementary Table 1). Cases with intracranial tuberculoma without associated meningitis were excluded from the analysis because this group could introduce bias due to their better prognosis than those with meningitis (data not shown). Level of consciousness was classified into three broad states: normal, lethargy/obnubilation, and stupor/coma, according to clinical assessment by the treating physician at admission.
Assessment of outcomes across the study periodsChanges in outcomes over time were evaluated in terms of mortality and neurological disability at 12-month follow-up after diagnosis. The study period was divided into four consecutive 7-year periods (1990–1996, 1997–2003, 2004–2010, and 2011–2017). Early mortality included deaths that occurred within the first two months, and late mortality included those that occurred between the second and twelfth months. Neurological disability was defined as any cognitive, sensorial, or sensitive-motor deficits that developed during the illness and remained at the 12-month follow-up.
Data analysisDifferences between the four study periods were assessed by the Student t-test or the Kruskal–Wallis test by ranks for continuous data, or by the χ2 for trend test for categorical data. Time to death was assessed using the Kaplan–Meier estimator, with follow-up censored at 12 months. The log-rank test was then used to compare the probability of survival between the four periods. The Cox proportional hazards model was used to perform univariate and multivariate survival analyses for patient survival, and the results are reported as hazard ratios (HR) and 95% confidence intervals (CIs). The proportionality of risks in the Cox model was verified using the Schoenfeld residuals. Differences in sequelae were assessed by univariate and multivariate logistic regression analysis and reported as odds ratios (OR) and 95% CIs. Covariables were entered in the adjusted analysis if they reached a significance level of at least 10% (p=0.10) and had no missing values. Sex and age were entered in the adjusted analysis regardless of the significance level in the univariate analysis. The level of significance was fixed at an alpha of 5%, using 95% confidence intervals for differences. Statistical analyses were performed with IBM SPSS version 25.0 (IBM Corp., Armonk, NY).
ResultsWe included 135 of 211 potential cases (Fig. 1). Twelve cases labeled as TBM were excluded because they corresponded to central nervous system (CNS) tuberculomas without concomitant meningitis. Except for minor variations on management, no significant differences were found between TBM cases included at the two sites (Supplementary Table 2).
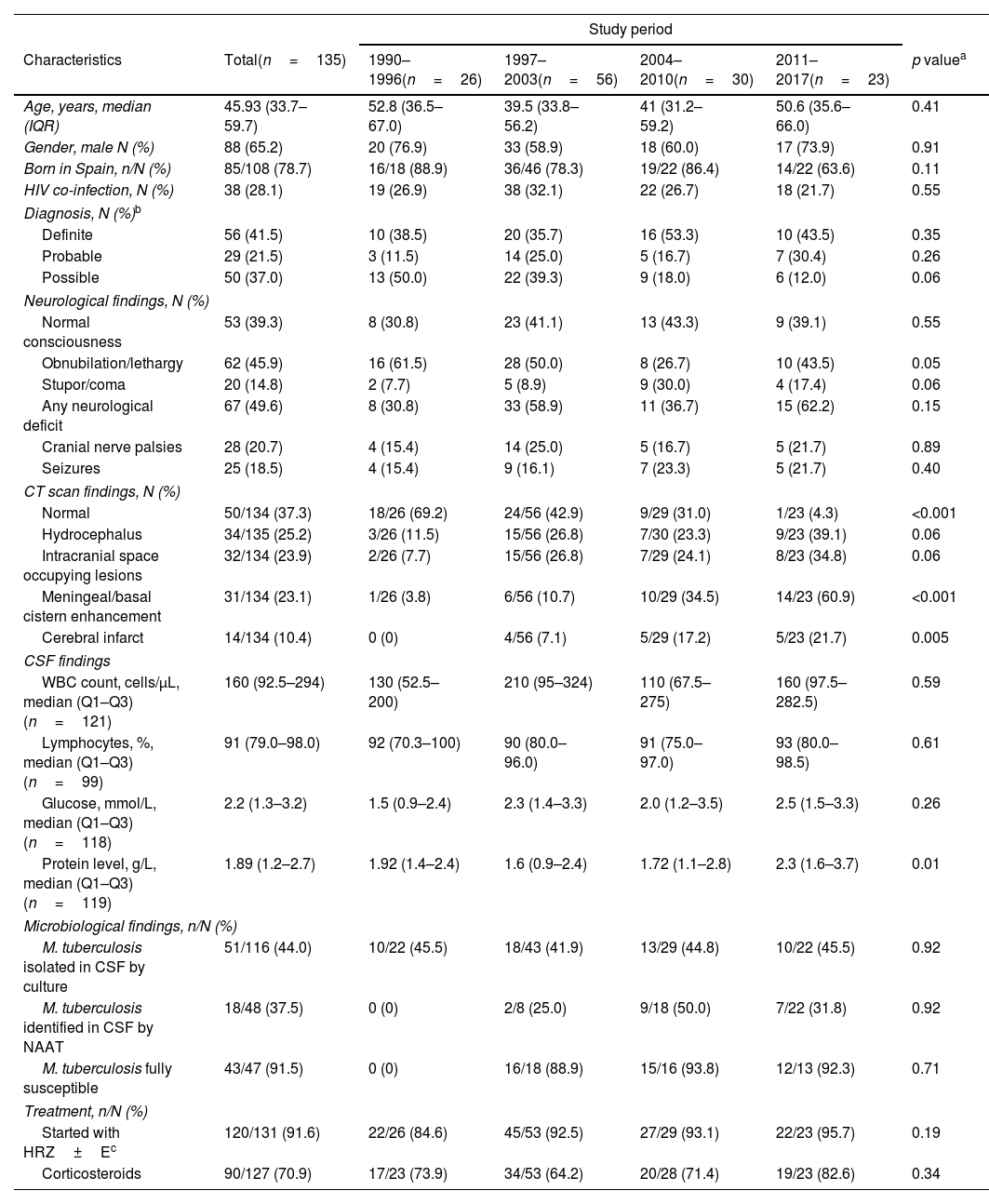
Most patients were born in Spain (78.7%), were predominantly male (65.2%), and had a median age of 47.6 years. HIV infection was present in 28.1%. On admission, 60.7% had low level of consciousness and 49.6% had some neurological deficit (except cranial nerves palsies). Overall, Mycobacterium tuberculosis complex was identified in the cerebrospinal fluid (CSF) by culture or nucleic acid amplification in 41.5%. There were trends toward a decrease in cases presenting with lethargy/obnubilation (p=0.05) and an increase in those presenting with stupor/coma over the study period (p=0.06) (Table 1).
Characteristics of 135 patients with tuberculous meningitis by study period.
| Study period | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Total(n=135) | 1990–1996(n=26) | 1997–2003(n=56) | 2004–2010(n=30) | 2011–2017(n=23) | p valuea |
| Age, years, median (IQR) | 45.93 (33.7–59.7) | 52.8 (36.5–67.0) | 39.5 (33.8–56.2) | 41 (31.2–59.2) | 50.6 (35.6–66.0) | 0.41 |
| Gender, male N (%) | 88 (65.2) | 20 (76.9) | 33 (58.9) | 18 (60.0) | 17 (73.9) | 0.91 |
| Born in Spain, n/N (%) | 85/108 (78.7) | 16/18 (88.9) | 36/46 (78.3) | 19/22 (86.4) | 14/22 (63.6) | 0.11 |
| HIV co-infection, N (%) | 38 (28.1) | 19 (26.9) | 38 (32.1) | 22 (26.7) | 18 (21.7) | 0.55 |
| Diagnosis, N (%)b | ||||||
| Definite | 56 (41.5) | 10 (38.5) | 20 (35.7) | 16 (53.3) | 10 (43.5) | 0.35 |
| Probable | 29 (21.5) | 3 (11.5) | 14 (25.0) | 5 (16.7) | 7 (30.4) | 0.26 |
| Possible | 50 (37.0) | 13 (50.0) | 22 (39.3) | 9 (18.0) | 6 (12.0) | 0.06 |
| Neurological findings, N (%) | ||||||
| Normal consciousness | 53 (39.3) | 8 (30.8) | 23 (41.1) | 13 (43.3) | 9 (39.1) | 0.55 |
| Obnubilation/lethargy | 62 (45.9) | 16 (61.5) | 28 (50.0) | 8 (26.7) | 10 (43.5) | 0.05 |
| Stupor/coma | 20 (14.8) | 2 (7.7) | 5 (8.9) | 9 (30.0) | 4 (17.4) | 0.06 |
| Any neurological deficit | 67 (49.6) | 8 (30.8) | 33 (58.9) | 11 (36.7) | 15 (62.2) | 0.15 |
| Cranial nerve palsies | 28 (20.7) | 4 (15.4) | 14 (25.0) | 5 (16.7) | 5 (21.7) | 0.89 |
| Seizures | 25 (18.5) | 4 (15.4) | 9 (16.1) | 7 (23.3) | 5 (21.7) | 0.40 |
| CT scan findings, N (%) | ||||||
| Normal | 50/134 (37.3) | 18/26 (69.2) | 24/56 (42.9) | 9/29 (31.0) | 1/23 (4.3) | <0.001 |
| Hydrocephalus | 34/135 (25.2) | 3/26 (11.5) | 15/56 (26.8) | 7/30 (23.3) | 9/23 (39.1) | 0.06 |
| Intracranial space occupying lesions | 32/134 (23.9) | 2/26 (7.7) | 15/56 (26.8) | 7/29 (24.1) | 8/23 (34.8) | 0.06 |
| Meningeal/basal cistern enhancement | 31/134 (23.1) | 1/26 (3.8) | 6/56 (10.7) | 10/29 (34.5) | 14/23 (60.9) | <0.001 |
| Cerebral infarct | 14/134 (10.4) | 0 (0) | 4/56 (7.1) | 5/29 (17.2) | 5/23 (21.7) | 0.005 |
| CSF findings | ||||||
| WBC count, cells/μL, median (Q1–Q3) (n=121) | 160 (92.5–294) | 130 (52.5–200) | 210 (95–324) | 110 (67.5–275) | 160 (97.5–282.5) | 0.59 |
| Lymphocytes, %, median (Q1–Q3) (n=99) | 91 (79.0–98.0) | 92 (70.3–100) | 90 (80.0–96.0) | 91 (75.0–97.0) | 93 (80.0–98.5) | 0.61 |
| Glucose, mmol/L, median (Q1–Q3) (n=118) | 2.2 (1.3–3.2) | 1.5 (0.9–2.4) | 2.3 (1.4–3.3) | 2.0 (1.2–3.5) | 2.5 (1.5–3.3) | 0.26 |
| Protein level, g/L, median (Q1–Q3) (n=119) | 1.89 (1.2–2.7) | 1.92 (1.4–2.4) | 1.6 (0.9–2.4) | 1.72 (1.1–2.8) | 2.3 (1.6–3.7) | 0.01 |
| Microbiological findings, n/N (%) | ||||||
| M. tuberculosis isolated in CSF by culture | 51/116 (44.0) | 10/22 (45.5) | 18/43 (41.9) | 13/29 (44.8) | 10/22 (45.5) | 0.92 |
| M. tuberculosis identified in CSF by NAAT | 18/48 (37.5) | 0 (0) | 2/8 (25.0) | 9/18 (50.0) | 7/22 (31.8) | 0.92 |
| M. tuberculosis fully susceptible | 43/47 (91.5) | 0 (0) | 16/18 (88.9) | 15/16 (93.8) | 12/13 (92.3) | 0.71 |
| Treatment, n/N (%) | ||||||
| Started with HRZ±Ec | 120/131 (91.6) | 22/26 (84.6) | 45/53 (92.5) | 27/29 (93.1) | 22/23 (95.7) | 0.19 |
| Corticosteroids | 90/127 (70.9) | 17/23 (73.9) | 34/53 (64.2) | 20/28 (71.4) | 19/23 (82.6) | 0.34 |
Abbreviations: ADA, adenosine deaminase; CNS, central nervous system; CSF, cerebrospinal fluid; CT, computed tomography; E, ethambutol; H, isoniazid; HIV, human immunodeficiency virus; IQR, interquartile range; n/N, number of cases with the condition/number for which the data was available; NAAT, nucleic acid amplification test; R, rifampicin; WBC, white blood cells; Z, pyrazinamide.
Of the 134 patients who initiated anti-tuberculous therapy, 97 (72.4%) completed their course, 22 (16.4%) died, and 15 (11.1%) were transferred to other centers during therapy. The intention was to treat 120 (89.6%) patients with a combination of rifampicin and isoniazid for 9 to 12 months, supplemented with pyrazinamide for the first 2 months, with or without ethambutol (standard treatment). Of them, 89 (74.2%) completed treatment, 21 (17.5%) needed modification, and treatment outcomes were unknown for 31 (of these, 13 died and 18 were transferred during therapy). Finally, 11 (8.2%) patients received other regimens (unknown in the other 3). Adjuvant corticosteroids were given in 90 (67.2%) cases for a median duration of 8 weeks (interquartile range, 5–12).
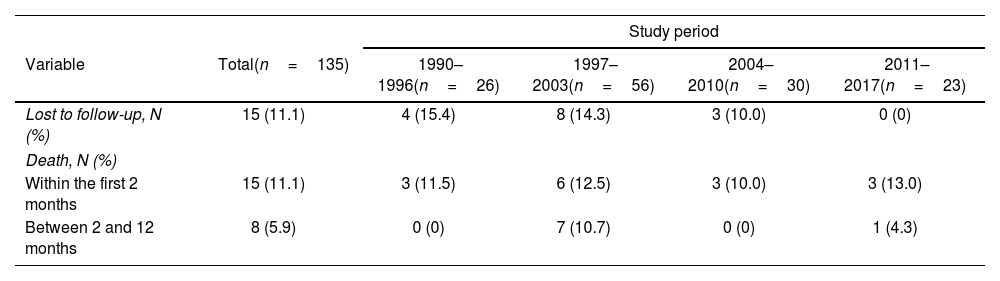
Changes in outcomes over timeIn total, 23 (17.0%) patients died. Of the 92 patients alive at 12-month follow-up, and for whom data were available, 13 (14.1%) had some sensory-motor disability, 12 (13.0%) had epilepsy, 4 (4.3%) had some cognitive disability, 3 (3.3%) had hearing loss, and 3 (3.3%) had speech disability. Overall, 57 (58.8%) patients died or suffered some neurological disability (Table 2).
Final status of 135 patients with tuberculous meningitis by study period.
| Study period | |||||
|---|---|---|---|---|---|
| Variable | Total(n=135) | 1990–1996(n=26) | 1997–2003(n=56) | 2004–2010(n=30) | 2011–2017(n=23) |
| Lost to follow-up, N (%) | 15 (11.1) | 4 (15.4) | 8 (14.3) | 3 (10.0) | 0 (0) |
| Death, N (%) | |||||
| Within the first 2 months | 15 (11.1) | 3 (11.5) | 6 (12.5) | 3 (10.0) | 3 (13.0) |
| Between 2 and 12 months | 8 (5.9) | 0 (0) | 7 (10.7) | 0 (0) | 1 (4.3) |
| Sequelae, N (%) | (n=92)a | (n=16)a | (n=35)a | (n=22)a | (n=19)a |
|---|---|---|---|---|---|
| Sensitive-motor disability | 13 (14.1) | 1 (6.3) | 5 (14.3) | 3 (13.6) | 4 (21.1) |
| Epilepsy | 12 (13.0) | 1 (6.3) | 4 (11.4) | 4 (18.2) | 3 (15.8) |
| Cognitive disability | 4 (4.3) | 0 (0) | 1 (2.9) | 1 (4.5) | 2 (10.5) |
| Hearing loss | 3 (3.3) | 1 (6.3) | 0 (0) | 0 (0) | 2 (10.5) |
| Speech disability | 3 (3.3) | 0 (0) | 0 (0) | 1 (4.5) | 2 (10.5) |
| Any neurological disability | 26 (28.3) | 2 (12.5) | 8 (22.9) | 7 (31.8) | 9 (47.4) |
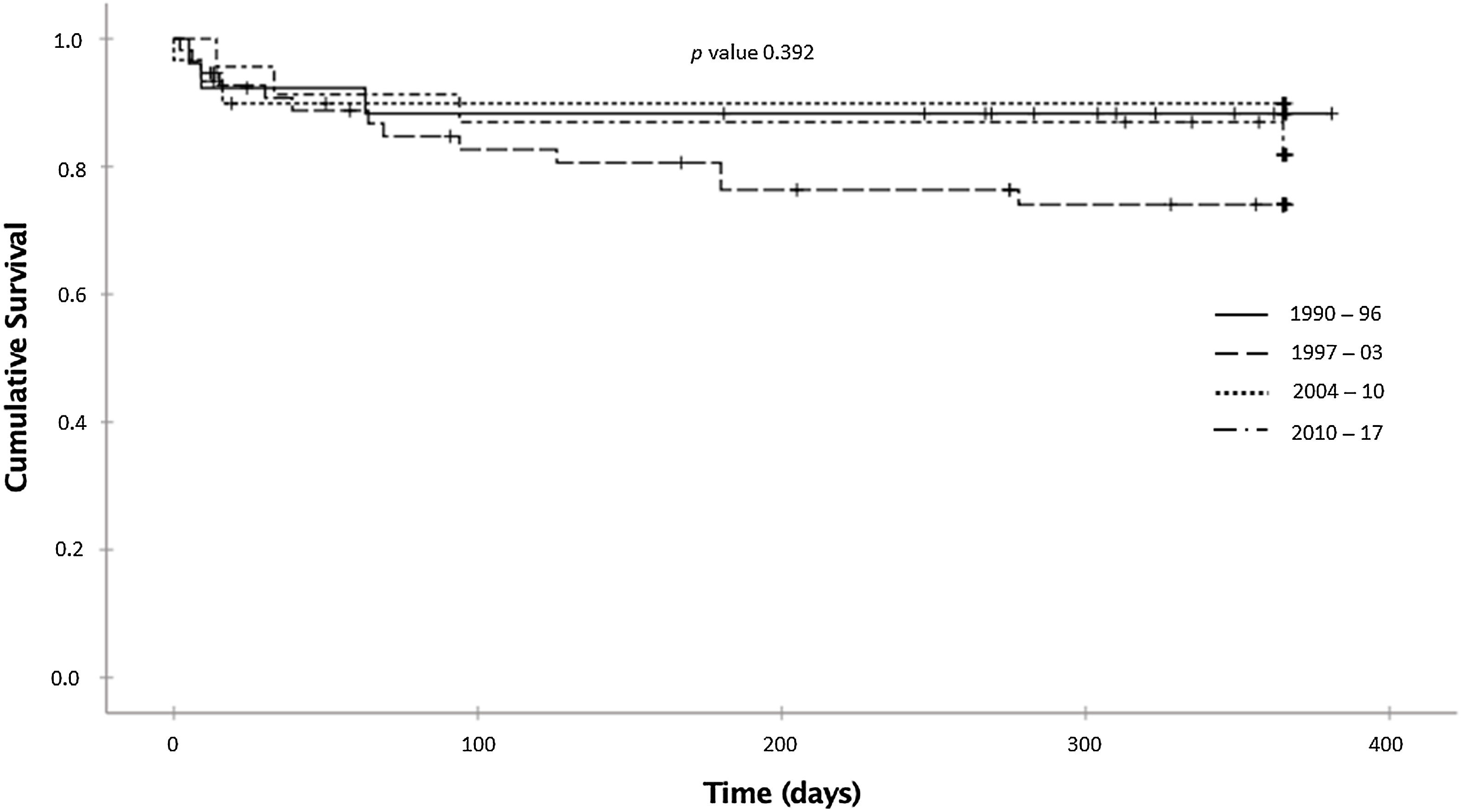
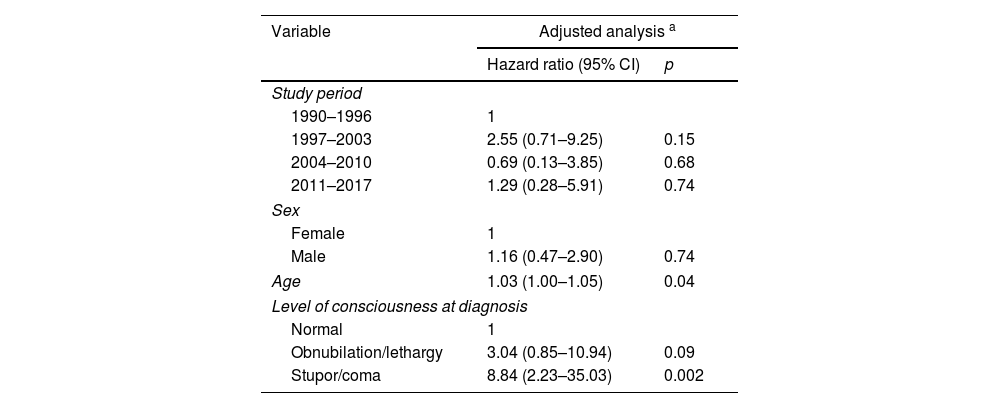
The probabilities of being alive at 12-month follow-up were 88.3%, 74.0%, 89.9%, and 81.9% in the 1990–1996, 1997–2003, 2004–2010, and 2011–2017 periods, respectively (p=0.392; log-rank test) (Fig. 2). After adjusting for covariates, no statistically significant differences were observed between the periods: compared with 1990–1996, the adjusted HRs were 2.55 (0.71–9.25) for the 1997–2003 period, 0.70 (0.13–3.85) for the 2004–2010 period, and 1.29 (0.28–5.91) for the 2011–2017 period. Age (adjusted HR 1.03 for each year older; 95% CI, 1.00–1.05) and level of consciousness (adjusted HR 8.84 for coma versus normal consciousness; 95% CI, 2.23–35.03) were independently associated with higher mortality (Table 3).
Adjusted Cox regression for mortality in 135 patients with tuberculous meningitis.
| Variable | Adjusted analysis a | |
|---|---|---|
| Hazard ratio (95% CI) | p | |
| Study period | ||
| 1990–1996 | 1 | |
| 1997–2003 | 2.55 (0.71–9.25) | 0.15 |
| 2004–2010 | 0.69 (0.13–3.85) | 0.68 |
| 2011–2017 | 1.29 (0.28–5.91) | 0.74 |
| Sex | ||
| Female | 1 | |
| Male | 1.16 (0.47–2.90) | 0.74 |
| Age | 1.03 (1.00–1.05) | 0.04 |
| Level of consciousness at diagnosis | ||
| Normal | 1 | |
| Obnubilation/lethargy | 3.04 (0.85–10.94) | 0.09 |
| Stupor/coma | 8.84 (2.23–35.03) | 0.002 |
Abbreviation: CI, confidence interval.
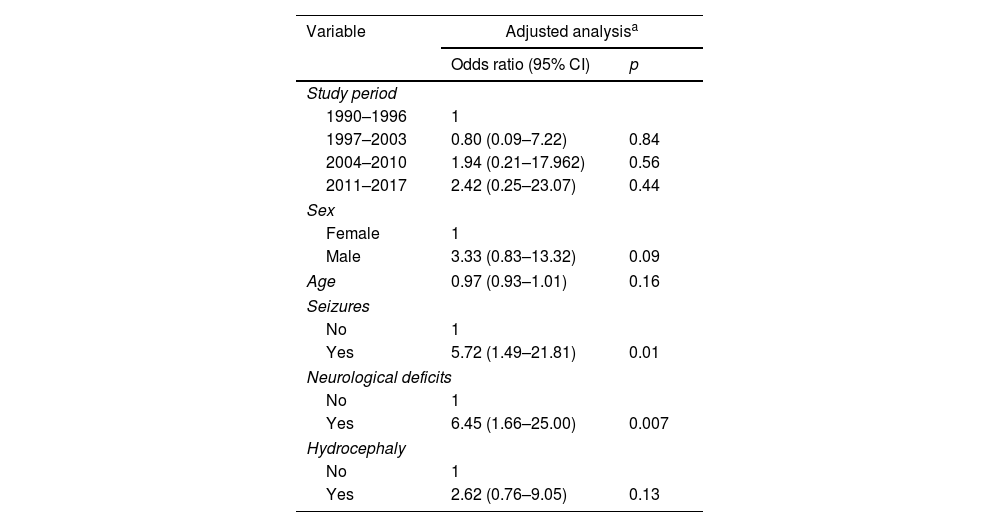
As for sequelae, we observed a progressive increase across the four periods (12.5%, 22.9%, 31.8%, and 47.4% in the 1990–1996, 1997–2003, 2004–2010, and 2011–2017 periods, respectively) (p=0.02) (Table 2). However, these differences disappeared after adjusting for covariates (versus the 1990–1996 period, the aORs were 0.80 (0.09–7.22) for the 1997–2003 period, 1.94 (0.21–17.96) for the 2004–2010 period, and 2.42 (0.25–23.07) for the 2011–2017 period. The presence of neurological deficits at diagnosis (adjusted OR 6.45; 95% CI, 1.66–25.00) and seizures (aOR 5.72; 95% CI, 1.49–21.81) were independently associated with a higher risk of sequelae (Table 4). The detailed crude and adjusted analyses for mortality and sequelae are given in Supplementary Tables 3 and 4.
Adjusted regression for sequelae in 135 patients with tuberculous meningitis.
| Variable | Adjusted analysisa | |
|---|---|---|
| Odds ratio (95% CI) | p | |
| Study period | ||
| 1990–1996 | 1 | |
| 1997–2003 | 0.80 (0.09–7.22) | 0.84 |
| 2004–2010 | 1.94 (0.21–17.962) | 0.56 |
| 2011–2017 | 2.42 (0.25–23.07) | 0.44 |
| Sex | ||
| Female | 1 | |
| Male | 3.33 (0.83–13.32) | 0.09 |
| Age | 0.97 (0.93–1.01) | 0.16 |
| Seizures | ||
| No | 1 | |
| Yes | 5.72 (1.49–21.81) | 0.01 |
| Neurological deficits | ||
| No | 1 | |
| Yes | 6.45 (1.66–25.00) | 0.007 |
| Hydrocephaly | ||
| No | 1 | |
| Yes | 2.62 (0.76–9.05) | 0.13 |
Abbreviation: CI, confidence interval.
This study shows that TBM-associated mortality and neurological sequelae have not improved in a high-resource setting, independently of diagnostic and therapeutic changes over time. A systematic review9 reported 25% mortality and 26–32% neurological disability, depending on the scale used, at 6-month follow-up. Furthermore, there was not a significant variation in mortality over time (12% in 1995–2000 and 31% in 2006–2010). Although both studies are not comparable, they also not show improvement in the prognosis of TBM over the last three decades.
Data from high-resource settings are scarce.10,12 In a study from New York City, 45% of patients with TBM that were susceptible to isoniazid and rifampicin died during follow-up, mostly within the first year after the diagnosis.10 Although the study provided no information about medical care coverage, healthcare access, and antiretroviral therapy, the reported 55% survival rate is clearly suboptimal. A recent multicenter study of children with TBM in Europe reported a 9.6% mortality at 12-month post treatment onset and that 52.9% of survivors had long-term sequelae.12
We identified age and a low level of consciousness at admission, which has been recognized as a poor prognosis factor, as risk factors for mortality. In our study, adjuvant corticosteroid therapy did not protect against mortality, probably because they were only administered to those patients with more severe disease before being formally recommended and to the lack of this data in a few clinical records. In our experience, mortality among HIV-infected patients was no worse than in the non-infected population, conflicting with previous reports. We consider that the universal access to healthcare and antiretroviral therapy in our country could explain this difference. Diagnostic delay, which could not be investigated, may also have contributed to the lack of improvement in prognosis by counteracting the benefits of improved diagnosis and management. It is conceivable that, as with other low-prevalence diseases, the level of suspicion and ability of clinicians to recognize TBM could have been gradually lost, leading patients being diagnosed at a more advanced stage of the disease.
In low-resource high-incidence TB settings, we might reasonably expect improvements in TBM outcomes by improving health care infrastructures, facilitating access, using the newest diagnostic tools, and guaranteeing antiretroviral therapy for patients with comorbid HIV. However, as the present results in a developed country illustrate, something more is needed. Rethinking antimicrobial therapy and using host targeted therapies are two approaches intended to improve the outcomes of TBM. Adding fluoroquinolones to the standard regimen did not benefit either mortality or disability outcomes (except in cases with monoresistance to isoniazid).5,13 The poor central nervous system penetration of rifampicin might also contribute to high mortality in TBM, and research has shown that doubling and triplicating the standard rifampicin doses can increase cerebrospinal fluid exposure without increasing adverse events.14–16 Although this approach also failed to show survival improvements, the relevant studies were not powered to demonstrate clinical benefit.5
Corticosteroids have been demonstrated to improve survival, but not disability of TBM.7,8 There is also encouraging evidence on the beneficial effect of tumor necrosis factor-alpha (TNFα) antagonists, which can successfully control the paradoxical reactions that occur despite corticosteroid therapy.17,18 Given the favorable experience and safety profile, the evaluation of TNFα antagonists in the context of optimized antimicrobial therapy for TBM is now warranted.
This study has limitations. First, the retrospective design might compromise data accuracy. Second, 24 of the 159 potential cases were excluded because essential data were unavailable; because these cases mostly corresponded to the earlier periods, their exclusion may have led to selection bias affecting our results, either over- or underestimating mortality. Third, the lack of data on the time elapsed between the onset of symptoms and diagnosis precluded adjusting the mortality analysis due to diagnostic delay. Finally, as therapeutic interventions were not standardized across time and sites, slight differences in care may limit our ability to find strong correlations with clinical outcomes.
This study, conducted in a high-resource low-incidence TB setting, shows that TBM continues to cause high mortality and disability, with no improvement observed since 1990.
FundingThis study had no specific financial support to be carried out. The A.S.M. work was supported by a postdoctoral grant “Juan Rodés” [JE18/00022] from the Instituto de Salud Carlos III through the Ministry of Economy and Competitiveness, Spain.
Conflict of interestThe authors have no relevant financial or non-financial interests to disclose.
To Natàlia Pallarés for statistical assessment.