Mycoplasma genitalium is a sexually transmitted pathogen responsible for 10-30% of non- gonococcal urethritis in men. In women, it is associated with cervicitis and complications such as pelvic inflammatory disease (PID) and possible infertility and poor obstetric outcomes1,2.
The lack of cell wall in M.genitalium precludes the use of ß-lactams and limits its treatment choice to antibiotics such as tetracyclines (doxycycline-DOX), macrolides (azithromycin-AZM) and quinolones (moxifloxacin-MXF). Due to the decrease in cure rates with DOX, AZM is the recommended first-line treatment against M.genitalium3,4. However, since Jensen et al. in 2008 reported AZM treatment failure due to single-nucleotide polymorphism (SNP) mutation at positions 2058 and 2059 (Escherichia coli numbering) in region V of the 23S rRNA gene5, the implementation of macrolide-resistance mutations (MRMs) assays has become of importance in order to minimize treatment failures. MXF is the second-line treatment recommended in such cases3,4.
To date, limited data has been published regarding the prevalence of AZM resistance-associated mutations in Spain6,7, so the objective of this study is to report the mutations found in the sanitary region of the province of Lleida between May 2019 and January 2021.
During this time, a total of 2288 specimens were tested for M.genitalium. DNA of the specimens was extracted using EZ1 or QIASymphony equipment (QIAGEN®), and real-time PCR screening was performed using the AllplexTM STI-7 V1-1 kit (Seegene®). Positive M.genitalium specimens were tested for MRMs with the Allplex MG&AziR (Seegene®) assay, which consists in a multiplex qPCR for detection of M.genitalium and six AZM SNPs (A2058G, A2058T, A2058C, A2059G, A2059T, A2059C). Both techniques were run on the CFX96 qPCR instrument (Bio-Rad®).
Of the 2288 of specimens, 46 samples from 46 patients (36 men and 10 women), consisting of 19 urethral swabs (41.3%), 14 first-void urines (30.4%), 10 endocervical swabs (21.7%) and 3 rectal swabs (6.5%), tested positive for M. genitalium, representing 2.1% of prevalence.
The request of sexually transmitted diseases (STD) testing of these samples were mainly urethritis (27) (58.8%), but also asymptomatic screening in high risk contacts (6) (13%), cervicitis (6) (13%), PID (4) (8.7%) and HIV pre-exposure prophylaxis (PrEP) (3) (6.5%).
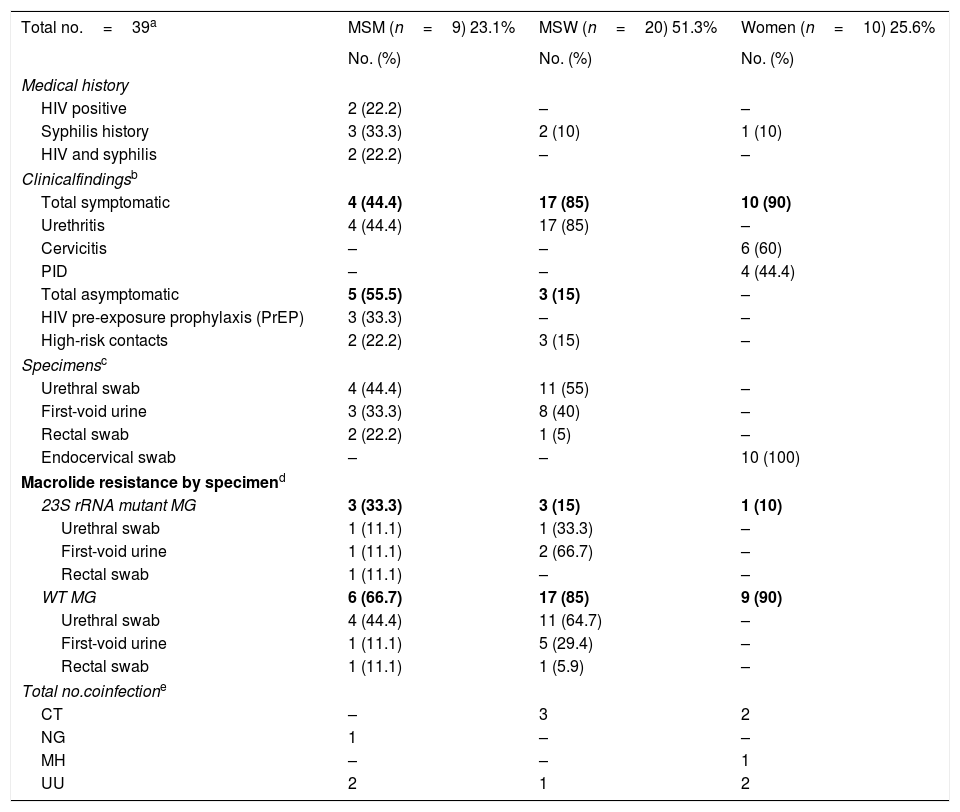
The demographic and clinical data of the 39 infection episodes are described and classified by the sexual orientation in Table 1. Among them, 20 were men who have sex with women (MSW) (51.3%), 9 were men who had sex with men (MSM) (23.1%), and 10 were women (25.6%). Seven medical records, all from men, were not available.
Demographic and clinical data of the positive M. genitalium episodes classified by the sexual orientation of patients.
| Total no.=39a | MSM (n=9) 23.1% | MSW (n=20) 51.3% | Women (n=10) 25.6% |
|---|---|---|---|
| No. (%) | No. (%) | No. (%) | |
| Medical history | |||
| HIV positive | 2 (22.2) | – | – |
| Syphilis history | 3 (33.3) | 2 (10) | 1 (10) |
| HIV and syphilis | 2 (22.2) | – | – |
| Clinicalfindingsb | |||
| Total symptomatic | 4 (44.4) | 17 (85) | 10 (90) |
| Urethritis | 4 (44.4) | 17 (85) | – |
| Cervicitis | – | – | 6 (60) |
| PID | – | – | 4 (44.4) |
| Total asymptomatic | 5 (55.5) | 3 (15) | – |
| HIV pre-exposure prophylaxis (PrEP) | 3 (33.3) | – | – |
| High-risk contacts | 2 (22.2) | 3 (15) | – |
| Specimensc | |||
| Urethral swab | 4 (44.4) | 11 (55) | – |
| First-void urine | 3 (33.3) | 8 (40) | – |
| Rectal swab | 2 (22.2) | 1 (5) | – |
| Endocervical swab | – | – | 10 (100) |
| Macrolide resistance by specimend | |||
| 23S rRNA mutant MG | 3 (33.3) | 3 (15) | 1 (10) |
| Urethral swab | 1 (11.1) | 1 (33.3) | – |
| First-void urine | 1 (11.1) | 2 (66.7) | – |
| Rectal swab | 1 (11.1) | – | – |
| WT MG | 6 (66.7) | 17 (85) | 9 (90) |
| Urethral swab | 4 (44.4) | 11 (64.7) | – |
| First-void urine | 1 (11.1) | 5 (29.4) | – |
| Rectal swab | 1 (11.1) | 1 (5.9) | – |
| Total no.coinfectione | |||
| CT | – | 3 | 2 |
| NG | 1 | – | – |
| MH | – | – | 1 |
| UU | 2 | 1 | 2 |
Considering coinfections with other STDs, 9M.genitalium cases (19.6%) were in coinfection with either Ureaplasma urealyticum (UU) (8.1%), Chlamydia trachomatis (CT) (6.9%), Neisseria gonorrhoeae (NG) (2.3%) and Mycoplasma hominis (MH) (2.3%).
Regarding MRMs, 37 episodes were classified as “wild type”/non-mutated (WT), whereas 9 cases (8 men and 1 woman) carried an AZM resistance mutation (3 urethral swabs, 4 first-void urines, 1 endocervical swab, and 1 rectal swab), showing a rate of mutation of 23%.
A2059G point mutation was detected most often (5/9) (55.6%), followed by A2058G (2/9) (22.2%), A2058T (1/9) (11.1%) and A2058C (1/9) (11.1%). From those, 3 mutations were found among MSM (3/9), 3 in MSW (3/20) and 1 in a woman (1/10). Two episodes with non-available medical records harboured a mutation (one urethral swab and one first-void urine).
In 6 of the patients harbouring an AZM SNP (66.6%), this macrolide was used up to a month before in different clinical processes (M.genitalium infection, cellulitis episode and chronic bronchitis).
Our results broaden MRMs prevalence in Spain and are similar to those found in Barcelona6, where the most prevalent mutation was A2059G (51.7%), followed by A2058G (41.4%), A2059C (3.4%) and A2058T (3.4%), with a total prevalence of 36%. In Gipuzkoa7, MRM prevalence was of 16.3%, but the most common one, over the total prevalence, was A2058G (8%), followed by A2059G (7.2%) and A2059C (0.4%).
Moreover, MRMs were more frequent among MSM (3/9) (33.3%) compared to MSW or women (15% and 10%, respectively), which has been previously reported6.
In conclusion, this study provides further evidence that macrolide resistance is highly prevalent in genitalium and supports the importance of MRMs detection in clinical laboratories to implement resistance-guided sequential therapy3. Additionally, the test of cure should be performed three weeks after antibiotic therapy to assess the treatment outcome8.
Conflict of interestThe authors declare no conflicts of interest.