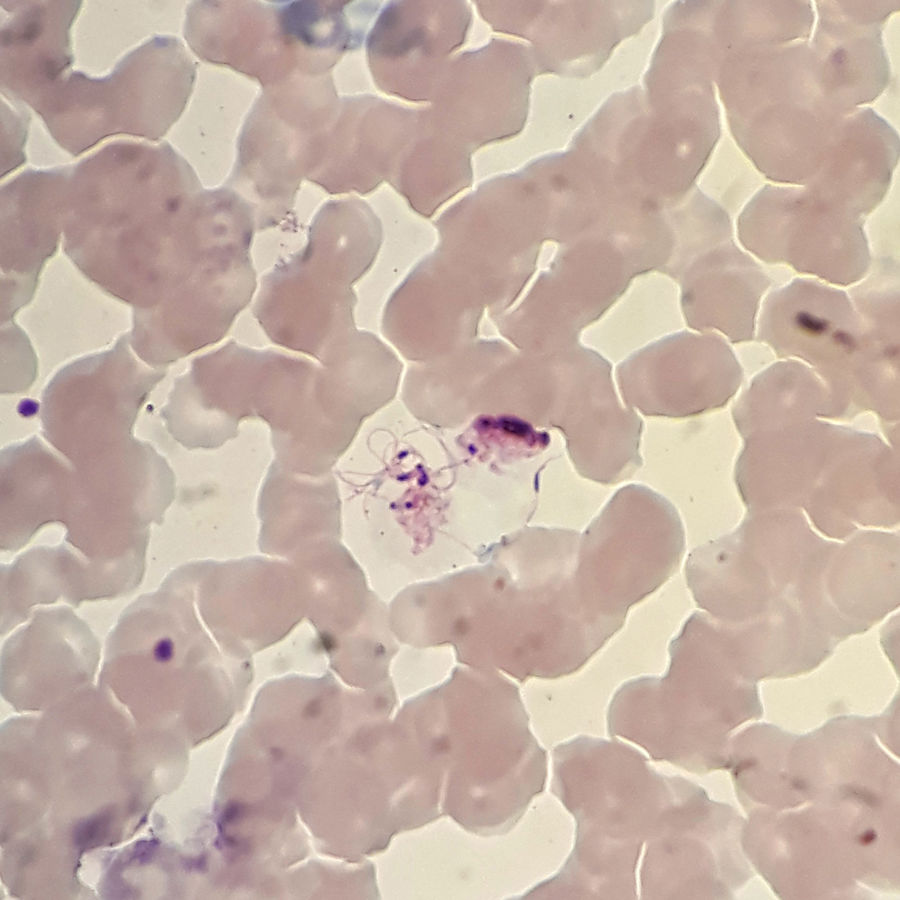
A 28-years-old male patient from India that had not travelled to his country in the last nine months presented to the emergency room with general discomfort, dysthermia feeling, sweating, chills, and shivering. In the physical examination, temperature was quantified in 40.5°C. No neurological deficit or peripheral lymphadenopathy was found. Blood was taken and sent to the local laboratory for examination and then sent to our hospital in order to confirm the diagnosis. This sample was stored for almost 3 days at room temperature.
Giemsa stain showed some strange forms, but the morphology of parasite was compatible with Plasmodium vivax. These forms were described like a structure smaller that a blood cell with peripheral flagella, a dark nucleus and mobile filament forms compatible with exflagellating microgametocytes (Fig. 1). The patient was treated with primaquine for two weeks and the infection was finally confirmed with PCR.
In the natural life cycle of the malarial parasites, exaflagellation of the microgametocyte, fertilization, and ookinetes production occur in the mosquito gut. The usual plasmodium forms seen in human peripheral blood smears include trophozoites, schizonts, macro, and microgametocytes. However, it has been demonstrated that exflagellation can occur ex vivo. Here a case of exflagellation in human blood is described. Exflagellation ex vivo is very uncommon but since 1880, Laveran had already noticed, while examining microscopically the blood of a patient suffering from malaria, “a body with mobile peripheral filaments” and “mobile filament freed for the body”.1 Exflagelation process was first described in 1884.2 Five years later, Golgi et al. saw few flagellate forms corresponding to those described by Laveran.3 In 1891, Romanowsky contributed with a staining method based on eosin and methylene blue that allowed seeing better these structures.4 Marchiafava et al. in 1892 also described “a form with flagella”.5 However, it was not until 1898 that Manson and Ross published that malaria organism had a second life, outside and independent of the human body.6 Nowadays, it is known that exflagellation of the microgametocyte occurs in the gut of mosquitoes, but if malaria blood is observed some time after it has been withdrawn from the blood vessels another form is seen, “the flagellated body”. By 1897, MacCallum discovered exflagellation and recognized its significance in the life cycle of malaria parasite. He described the fertilization process and recognized the flagellum as the male gamete.7 After that, there are several references to this phenomenon.8
According to some authors, exflagellation can be induced in vitro by dropping the temperature of cultured gametes from 37°C to 28°C, thus simulating the change from host to vector temperatures, by either increasing the pH from 7.5 to 8.2 or by the addition of a gametocyte-activating factor (GAF), the oxanthurenic acid. This compound has been isolated from the mosquito gut and is supposed to be implicated in the maturation of the microgametocytes.9 In this case, probably a delay in sample processing and a dropping of the temperature could be the cause of exflagellation process. Fertilization has been shown to take place, at least in vitro conditions, almost immediately following exflagellation within 10 to 20min after the initiation of gametogenesis.10
The experimental in vitro phenomenon of exflagellation is very common, but it is rarely described in the clinical setting of malaria. Nevertheless, it is important to consider it when atypical structures are found in the blood from a patient with suspected malaria to avoid mistake in the diagnosis.
FundingsNone.
Conflict of interestThe authors declare no conflict of interest.