This was a 28-year-old patient from Portugal who had been in Spain for 15 days and had sexual relations with men (men who have sex with men [MSM]). He went to the Accident and Emergency department with diarrhoea and a fever of 38.1°C, plus mild dysuria and leucocytosis of 20×103μl in blood. Stool and urine samples were collected, and the patient was admitted to the hospital and given intravenous ceftriaxone (1g/24h) as empirical treatment. Shigella spp. was isolated in the stool culture, and the patient was discharged with oral ciprofloxacin (500mg/12h for 3 days) before the susceptibility to antimicrobials had been determined. The isolate turned out to be a group 9 CTX-M extended-spectrum β-lactamase (ESBL)-producing Shigella sonnei (S. sonnei), resistant to cephalosporins, cotrimoxazole and quinolones. Several days later, the patient was admitted again due to persistent symptoms of high fever and watery diarrhoea, this time being treated with ertapenem (1g every 24h intravenously for 5 days). He was discharged at the end of the course of antibiotics due to resolution of the symptoms.
Case No.2This was a 47-year-old patient, MSM, taking ongoing treatment with pre-exposure prophylaxis (PrEP) who attended the sexually transmitted infection (STI) clinic for follow-up and to receive prophylaxis with ceftriaxone (1g intramuscularly) due to risky sexual contact. He reported a 48-h history of symptoms of diffuse abdominal pain with increased bowel movements (up to 10 per day) with pathological products (mucus/blood), but at that point a watch and wait approach was adopted. He was seen again seven days later and, as the symptoms persisted, stool culture samples were collected from the patient and his current partner (with similar gastrointestinal symptoms) and active monitoring was continued while awaiting the results. In the stool sample, group CTX-M-9, cephalosporinase-type ESBL-producing S. sonnei was detected, while non-ESBL-producing Shigella flexneri (S. flexneri), whose sensitivity to antimicrobials was different, was detected in its partner (only resistant to quinolones).
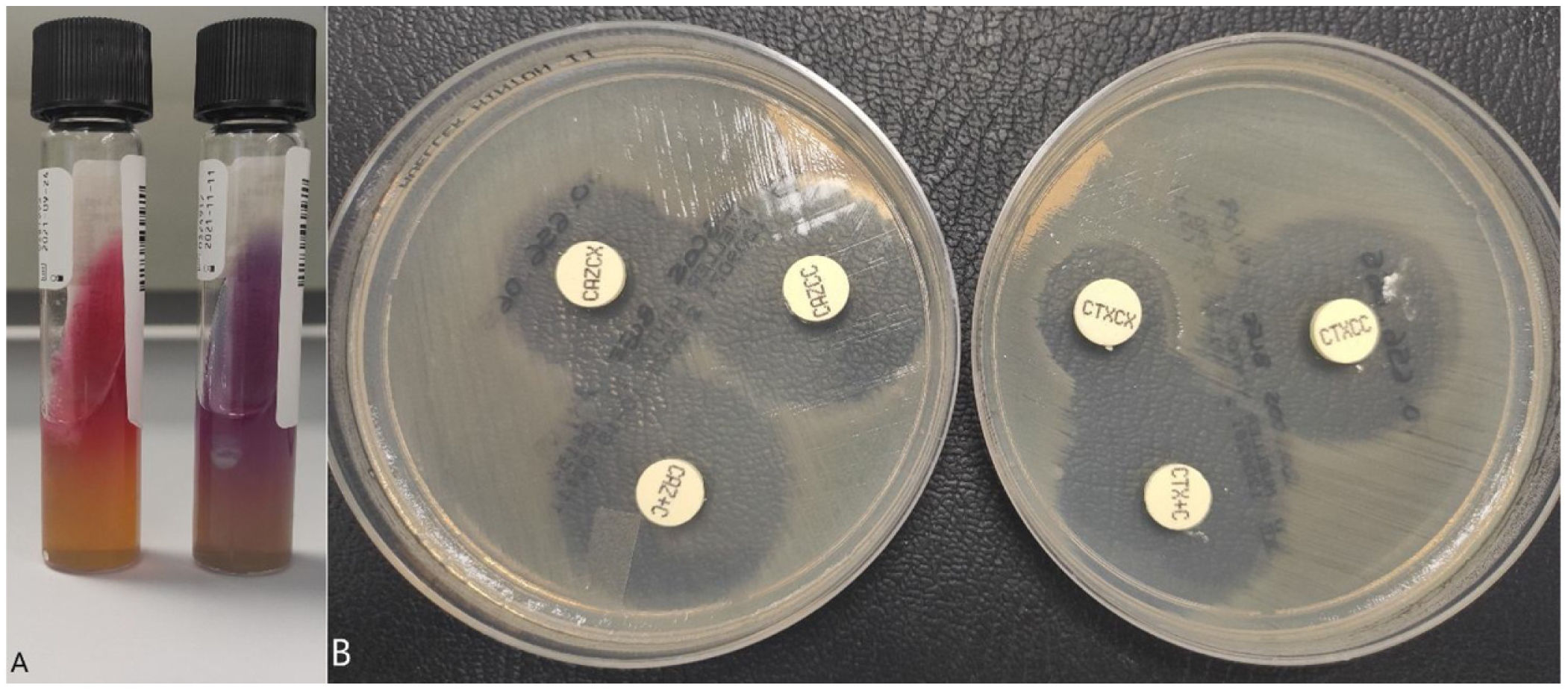
MicrobiologyIn the first case, a PCR panel "STI Essential Assay, Allplex™" (Seegene Inc., Seoul, South Korea) was performed on the urine sample (first-catch sample) with detection of Chlamydia trachomatis. In both cases, a stool sample was extracted to perform the "Enteric bacterial" PCR panel of the BD Max™ system (Becton Dickinson, Franklin Lakes, NJ, USA) with detection of enteroinvasive Shigella/Escherichia coli (E. coli) DNA. Subsequently, the stool sample was seeded on MacConkey and Hektoen (BD™) agar media, with lactose-negative colonies isolated on the MacConkey agar which were identified as Shigella spp./E. coli by MALDI-TOF mass spectrometry (Bruker, Massachusetts, USA); this system is not able to distinguish between these two genera. Lactose-negative colonies were plated on triple sugar iron (TSI) agar and lysine iron agar (LIA) (BD™) media for identification, confirming the genus Shigella spp. (Fig. 1A). Agglutination was then performed with "Difco™ Shigella Antisera Poly" (BD™) to determine the species, and the identification of S. sonnei was confirmed.
Identification of Shigella spp. and detection of the presence of an extended-spectrum beta-lactamase (ESBL).
(A) Left: TSI (triple sugar-iron) medium, glucose fermentation can be seen but not producing gas; right: LIA (lysine-iron agar) medium, there is no decarboxylation or deamination of the lysine. (B) Disc-diffusion technique with the confirmation kits "Total ESBL, AmpC and ESBL+AmpC Confirm kit" (ROSCO Diagnostica A/S, Taastrup, Denmark). The increase in the cefotaxime and ceftazidime halo (>5mm) can be seen in the presence of clavulanic acid but not in cloxacillin, confirming the presence of ESBL.
The antibiogram was performed using the disc-diffusion technique, as well as the "ID/NMIC 503" panel of the Phoenix BDR system (Becton Dickinson, Franklin Lakes, NJ, USA), through which resistance was detected to cephalosporins, aztreonam, trimethoprim/sulfamethoxazole and quinolones. Susceptibility to azithromycin was also studied in both isolates using E-testR, showing resistance with MIC>256mg/l (epidemiological cut-off point for wild strains at ≤16mg/l). We performed a rapid immunochromatography test ("NG-Test CTX-M Multiple"; NG Biotech, Guipry–Messac, France), which enables detection of the most common types of cephalosporinases (groups CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9 and CTX-M-25), and the result was positive. The ESBL resistance phenotype was then confirmed using the disc-diffusion technique with the confirmation kits (Fig. 1B) "Total ESBL, AmpC and ESBL+AmpC Confirm kit" (ROSCO Diagnostica A/S, Taastrup, Denmark) and the "Check-Direct ESBL Screen" panel of the BD Max™ system with detection of group 9 CTX-M cephalosporinase-type beta-lactamase (which can include different genes: blaCTX-M-14, blaCTX-M-24 and blaCTX-M-27).
Both strains were sent to the National Centre for Microbiology (Instituto de Salud Carlos III, Majadahonda, Madrid, Spain) which performed phenotypic and genotypic detection of antibiotic resistance. Both strains were detected as being carriers of CTX-M-27 ESBL by phenotypic techniques and by sequencing, belonging to the 152 sequence (MLST). The analysis of the genomes indicated that both isolates belonged to the cluster from the multi-drug resistant S. sonnei alert issued by the United Kingdom in the months prior to the exposed cases.1 The two isolates were identical to each other within 0 alleles using the cgMLST scheme of the SeqSphere software (Ridom Bioinformatics, Germany).
DiscussionThe genus Shigella (includes the species S. sonnei, S. flexneri, Shigella boydii and Shigella dysenteriae) causes a gastrointestinal syndrome called shigellosis. Most patients develop diarrhoea (sometimes with blood and/or mucus), fever and abdominal pain. Transmission occurs mainly by person-to-person contact with contaminated food or water. The infection is normally self-limiting, without the need for antibiotic treatment. As far as sexual transmission is concerned, in recent years it has been associated with outbreaks among MSM throughout the world, in the USA and Spain and in London in the UK.2–4 Regarding infection risk factors, it is possible that the use of PrEP could be influencing sexual behaviour by reducing the use of barrier measures (condoms), thus increasing the prevalence of STI, including shigellosis as an emerging cause.5
Resistance to antimicrobials due to ESBL is becoming a major problem. There have been very few reports to date of ESBL-producing S. sonnei strains in patients who have not travelled to endemic areas (Asia), so an adequate surveillance programme could provide us with information about the epidemiological situation here in Spain.6–8 Specifically in the UK, several outbreaks (or a prolonged outbreak) of ESBL-producing S. sonnei (blaCTX-M-27-Group 9 gene) and a QnrB19 plasmid (reduced sensitivity to quinolones) have been reported among MSM since 2015.9 In Australia an increase has been noted since 2019 in cases of shigellosis among MSM whose strains share a profile similar to those studied in the UK (probably introduced by travellers who returned from an area where this clonal group was circulating).10 In Spain, there are eight confirmed cases and 22 possible cases related to these multi-drug resistant strains of S. sonnei. In fact, at least four other sequenced isolates, and our two strains, are closely related within the cluster and to representative sequences from the UK.1
In MSM patients with gastrointestinal symptoms including fever, abdominal pain, vomiting or diarrhoea with pathological products such as blood or mucus, Shigella spp. should be suspected as a sexually transmitted infection. The use of antibiotics should be restricted to moderate/severe cases to avoid the consolidation and transmission of antibiotic resistance. From an epidemiological point of view, transmission should be monitored and prevention measures should be taken as with any other STI, avoiding sexual intercourse and doing a repeat stool culture to ensure that the bacterial load is low or undetectable.
FundingNo funding was received for this study.