The genera Phlebovirus transmitted by Diptera belonging to the Psychodidae family are a cause of self-limited febrile syndrome in the Mediterranean basin in summer and autumn. Toscana virus can also cause meningitis and meningoencephalitis. In Spain, Toscana, Granada, Naples, Sicily, Arbia and Arrabida-like viruses have been detected. The almost widespread distribution of Phlebotomus genus vectors, and especially Phlebotomus perniciosus, in which several of these viruses have been detected, makes it very likely that there will be regular human infections in our country, with this risk considered moderate for Toscana virus and low for the other ones, in areas with the highest vector activity. Most of the infections would be undiagnosed, while only Toscana virus would have a greater impact due to the potential severity of the illness.
Los virus del género Phlebovirus, transmitidos por dípteros de la familia Psychodidae, son una causa de síndrome febril autolimitado durante el verano-otoño en los países mediterráneos. En el caso del virus Toscana, pueden ser causa de meningitis y meningoencefalitis. En España se ha detectado la presencia de los virus Toscana, Granada, Nápoles, Sicilia, Arbia y Arrabida-like. La presencia casi generalizada de vectores del género Phlebotomus, especialmente de Phlebotomus perniciosus, en los que se han detectado varios de estos virus, hace muy probable que aparezcan de manera regular infecciones en humanos en nuestro país, siendo este riesgo moderado para el virus Toscana y bajo para el resto, en las zonas con mayor actividad del vector. La mayor parte de las enfermedades pasarían inadvertidas y solo el virus Toscana puede tener un mayor impacto por la aparición de casos graves.
The Phlebovirus genus belongs to the Phenuiviridae family and Bunyaviridae order. This genus comprises 10 species and more than 100 antigenically different viruses and is in turn divided into two groups: those transmitted by sandflies and the Uukuniemi group, transmitted by ticks. The sandfly-borne phleboviruses (SBPs), which circulate in the Mediterranean region, are summarised in Table 1.
Sandfly-borne phleboviruses, pathogens (or potentially pathogens) for humans in the Mediterranean region.
| Species | Virus |
|---|---|
| Naples | Naples, Toscana, Massilia, Tehran, Granada, Punic, Fermo, Saddaguia, Arrabida, Zerdali |
| Salehabad | Salehabad, Arbia, Adria, Alcube, Edirne, Adana, Medjerda Valley |
| Sicily | Sicily, Cyprus, Turkey, Utique |
| Corfu | Corfu, Sicily-like, Gime 1, Gime 2, Olbia, Provencia |
| Karimabad | Karimabad |
In general, SBP infections are asymptomatic and sometimes cause a 3–7 day febrile syndrome called “Pappataci fever” with flu-like symptoms. The incubation period for SBPs in an infected person is usually short, about 3–6 days,4 and the onset is abrupt with fever, myalgia, headache, conjunctivitis, nausea, vomiting and diarrhoea, which generally resolve within a week. Leukocytopaenia, thrombocytopaenia, and elevated liver enzymes and creatine kinase are also common.5 The Granada virus could also be associated with febrile syndromes with rash or upper respiratory infection.6
Neuroinvasive disease has been described almost exclusively associated with Toscana virus infection.7 In these cases, clinical presentation may include, in addition to the aforementioned symptoms, photophobia, nystagmus, neck stiffness, Kernig's sign, focal neurological deficit and altered level of consciousness. Lymphocytic pleocytosis with 5–10cells/ml can be found in cerebrospinal fluid (CSF), with normal levels of glucose and proteins, which is characteristic of aseptic meningitis. The prognosis is generally good, without sequelae, although cases of severe encephalitis, persistent headache, hearing loss, permanent personality changes, prolonged low level of consciousness with seizures, hydrocephalus and persistent aphasia or paresis have been described in the months after the acute phase.7–13 Other serious complications described are epididymo-orchitis, disseminated intravascular coagulation and haemorrhagic manifestations.12
The most common case detection, in the case of the Toscana virus, is sporadic. The rest of the SBPs, due to the symptoms being mild, are usually detected only when they present in the form of flare-ups. To date, they are considered to be exclusively human diseases.5
SBPs can be detected by culture in certain cell lines, such as Vero cells (African green monkey kidney, Cercopithecus aethiops) and LLC-MK2 (Rhesus, Macaca mulatta), as well as in BHK-21 cells (hamster kidney), with Vero cells the most utilised.5 There are molecular techniques that can detect low concentrations of the virus and that are designed ad hoc for specific viruses. The most widely used technique for the molecular diagnosis of these viruses is currently the real-time polymerase chain reaction (RT-PCR), which is more sensitive and faster. It must be taken into account that, due to the genetic variability of the virus, these techniques may not be very sensitive for the detection of strains that circulate in other areas.13 The detection of specific IgM and IgG antibodies is performed using enzyme-linked immunosorbent techniques (ELISA) or indirect immunofluorescence (IFI), in serum and CSF. Although the sensitivity of these techniques is very high, cross-reactions between the different phleboviruses can occur since they are antigenically similar, especially when using IFI techniques. Serum neutralisation tests are performed to confirm the serological diagnosis.13,14
There are some effective agents against SBPs in vitro. In this regard, selenazole, ribavirin, interferon alpha and interferon-derived MxA protein are inhibitors of the Sicilian virus and pyrazine derivatives T-705 and T-1106 are inhibitors of the Naples virus. Ribavirin has been used successfully as post-exposure prophylaxis in soldiers exposed to the Sicilian virus.5 There is no specific treatment for the Toscana virus. At the moment, there are no vaccines against phleboviruses. Prevention at the individual level is based on the use of measures to avoid bites, mainly in the warmer months of the year, when there is a greater risk of transmission.
Disease transmission and life cycleThe only known mechanism of transmission of SBPs is vectorial and the main competent vectors are sandflies belonging to the Diptera order and Psychodidae family. The genera involved in the transmission are Lutzomyia, in the American continent, and Phlebotomus in Europe, mainly the species Phlebotomus perniciosus (P. perniciosus), Phlebotomus ariasi (P. ariasi), Phlebotomus papatasi (P. papatasi) and Phlebotomus perfiliewi (P. perfiliewi).
The biological cycle of sandflies consists of 4 stages: egg, larva, pupa and adult, and it can take about two months to complete under optimal conditions. The female lays 4–5 clutches throughout her life, with about 40–60 eggs in each clutch. Characteristic places for oviposition are moist and well-sheltered places such as holes and cracks in walls, deteriorated masonry, deep cracks in the ground and burrows.15 The adult insect has a short life, rarely more than 4 weeks. It forages for food within a radius of about 50m from where it breeds, although it can occasionally move greater distances. In general, it travels in short flights, making small jumps. Unlike the mosquito, it is very quiet in flight. It is active once the sun has set and during the first hours of the night, when the female usually bites both inside and outside houses, depending on the species. Sandfly activity is highest in the warmer months, between June and October.16,17
Transmission of the virus to humans and animals occurs through the bite of an infected sandfly. In these insects, the virus replicates and spreads to the salivary glands. It is transmitted to different animals via the bite of the females. Reverse transmission occurs when the female sandfly feeds on the blood of infected animals or humans in the viremic period.18 SBPs have been found in both male and female sandflies, suggesting the existence of transovarian or vertical transmission. This mechanism is of utmost importance, since it would allow the survival of the virus in subsequent generations of sandflies. Experimental infection of females via the venereal route has also been demonstrated, which would be a way of amplifying the infection in the vector in the absence of other reservoirs.19 Sandflies become infective about seven days after acquiring the virus and remain so throughout their lives.4
Sandflies are considered to act as the main reservoir of the virus. Antibodies against SBPs have been detected in various domestic animals such as dogs, cats, goats and sheep in different parts of the Mediterranean basin. However, the only SBP that has been detected in animal blood is the Toscana virus, although viraemia appears to be short-lived, so their role as reservoirs is still contested. In symptomatic humans, it has been observed that viraemia is short, which, as would occur with animals, makes their action as reservoirs unlikely,6,20 although in the case of the Naples virus this possibility has recently been indicated using estimates made with mathematical models.21
Based on these data, both animals and humans could be considered accidental hosts, serving as a temporary refuge and as a vehicle to access the main reservoir, the sandfly. Vertebrates would thus increase the chances of survival and transmission of these viruses.
Vector control measuresThe vector control programmes that are developed as part of the prevention strategies of the authorities, both local and national, have an impact on the density of vectors. In general, these programmes are a very effective measure for reducing or interrupting the transmission of infections. However, in the specific case of sandflies, they are not very effective, are carried out only sporadically, and are mainly aimed at controlling leishmaniasis, the vector of which is also the sandfly. On the one hand, the habits of the females of most of the species found in Spain are exophagic (they bite outdoors) and exophilic (they rest outdoors during the maturation stage of the eggs), which makes insecticides applied indoors less effective. On the other hand, sandfly populations can be found in different areas and the action of pyrethroid insecticides on larval stages is nil or very limited due to the inaccessibility of the few known breeding habitats and the difficulty of determining precisely where they are actually developing. In other words, these populations are distributed in very wide areas, where there are a large number of potential refuges and breeding areas of a very varied typology. Due to these factors, the effects of disinsection treatments can produce contradictory results and are very limited in time. In some contexts, environmental treatments by nebulisation or thermospray with pyrethroid insecticides have been effective in areas with special vector density and risk of transmission due to their proximity to inhabited places (mainly interurban parks) and also the spraying of pyrethroid insecticides with higher residual capacity on walls, inspection chambers, plots of land, farms, culverts, etc., in areas close to homes or even inside the urban area.22 It must be taken into account that the growing resistance to insecticides, fortunately not very widespread in sandflies, and the gradual reduction in the number of insecticides authorised and marketed in the European Union for use in preventive vector control programmes significantly reduces the tools available for prevention.23
Other environmentally-focused actions have proven to be effective in vector control, such as habitat destruction and modification (e.g. plastering of cracks in walls and pavements, and removal and covering of rocks), creating physical barriers to prevent vector activity (e.g. closing and sealing storm drains and wells, and closing or meshing basement air vents), and limiting areas of refuge and feeding (clearing and removing excessive vegetation and stacks of firewood, plant debris and other organic debris, cleaning sewers, and pest control of animals that could be potential food sources for the vector).22
The sandfly and phlebovirus situation in SpainEleven species of sandflies have been identified in Spain belonging to two genera: Sergentomyia and Phlebotomus. Although P. perniciosus and P. ariasi are the most widespread species of sandflies in Spain, others, such as P. papatasi and Phlebotomus sergenti are distributed to a greater or lesser extent throughout the country. The main distribution of these vectors is in the south and centre of the Iberian peninsula and the Mediterranean coast, as well as the Balearic Islands. The presence of P. perfiliewi, the most abundant transmitter of the Toscana virus in Italy has not been documented in Spain. Other species such as Phlebotomus langueroni, Phlebotomus mascitti, Phlebotomus alexandri and Phlebotomus chaubadi are found occasionally, while Phlebotomus similis, Phlebotomus tobbi and Phlebotomus neglectus are not present in Spain.24–28 In the Canary Islands, the genus Phlebotomus has a scarce presence, P. perniciosus and P. ariasi have been found at very low densities, as well as Phlebotomus fortunatarum, an endemism of these islands.29
The density of sandflies varies depending on the weather and land use. In many areas of Spain, populations, especially those of P. perniciosus, have two peaks of abundance, one in spring and early summer, and one in autumn. The autumn peak is certainly more important in the transmission of SBPs because a higher percentage of females that have previously ingested blood are detected and, therefore, the possibility of being infected will be higher. In the province of Madrid, the presence of P. perniciosus was detected from May to November with two peaks of abundance, in July and in September. In the same area, P. ariasi showed activity from May to October, but with a peak of abundance only in August. In Spain, it has been observed that the density of P. perniciosus is greater in the driest and most arid areas with warmer temperatures, predominantly in the so-called meso- and thermo-Mediterranean areas. On the other hand, P. ariasi is better adapted to colder areas and altitudes above 600–900m (supra-Mediterranean areas).24–26,30 Regarding soil usage, P. perniciosus and P. ariasi predominate in non-urban agricultural areas, without paved roads, in walls protected from the wind (especially on the south and east faces), and in areas where there are livestock and birds.31 In the risk maps carried out in the study in the province of Granada, it was found that the likelihood of P. perniciosus being present increased in buildings with older construction dates.25,26
In Spain, there are indications of the presence of the Toscana, Granada, Naples, Sicily, Arbia and Arrabida-like viruses in humans, animals and sandflies, although only human cases associated with the Toscana virus have been identified (Tables 2 and 3).
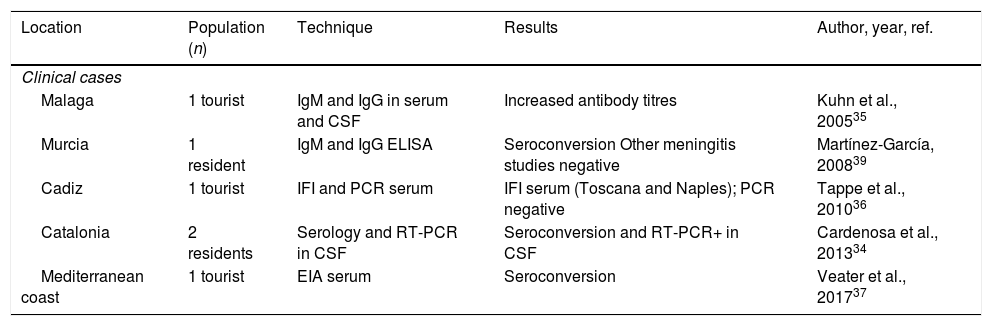
Human cases of aseptic meningitis due to Toscana virus in Spain.
| Location | Population (n) | Technique | Results | Author, year, ref. |
|---|---|---|---|---|
| Clinical cases | ||||
| Malaga | 1 tourist | IgM and IgG in serum and CSF | Increased antibody titres | Kuhn et al., 200535 |
| Murcia | 1 resident | IgM and IgG ELISA | Seroconversion Other meningitis studies negative | Martínez-García, 200839 |
| Cadiz | 1 tourist | IFI and PCR serum | IFI serum (Toscana and Naples); PCR negative | Tappe et al., 201036 |
| Catalonia | 2 residents | Serology and RT-PCR in CSF | Seroconversion and RT-PCR+ in CSF | Cardenosa et al., 201334 |
| Mediterranean coast | 1 tourist | EIA serum | Seroconversion | Veater et al., 201737 |
| Retrospective studies in aseptic meningitis series | ||||
| Granada | Cases between 1988 and 1996 (n: 184) | CSF culture | 15 positives (8%) | Mendoza-Montero et al., 199843 |
| Spain | Cases between 1993 and 1999 (n: 81) | EIA IgG and IgM in serum and CSF | 7 positives (8.6%) | Echevarría et al., 200341 |
| Spain | Cases between 1988 and 2002 (n: 724) | CSF culture | 17 positives (2.3%) | Navarro et al., 200438 |
| Spain | Cases between 2000 and 2005 (n: 382) | IFI serum | 19 positives (4.9%) | De Ory et al., 200944 |
| Mallorca | Cases between 2007 and 2008 (n: 22) | RT-PCR CSF | 0 positives | Leyes et al., 201142 |
EIA: enzyme immunoassay; IFI: indirect immunofluorescence; IgG: immunoglobulin G; IgM: immunoglobulin M; CSF: cerebrospinal fluid; PCR: polymerase chain reaction.
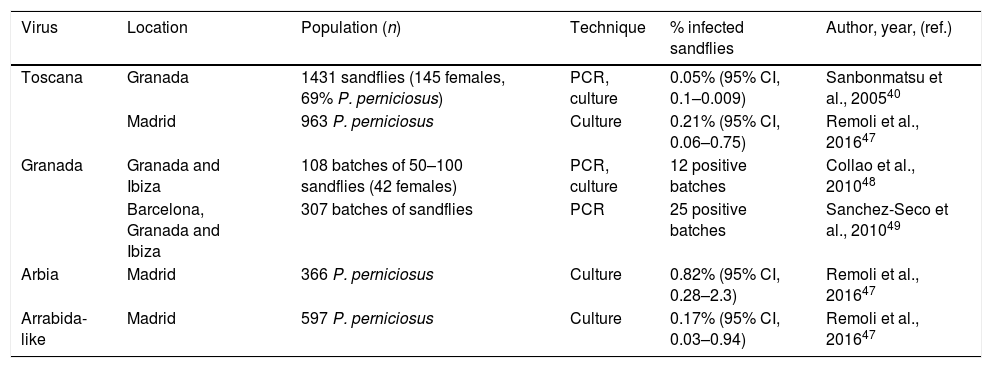
Detection of phlebovirus in sandflies in Spain.
| Virus | Location | Population (n) | Technique | % infected sandflies | Author, year, (ref.) |
|---|---|---|---|---|---|
| Toscana | Granada | 1431 sandflies (145 females, 69% P. perniciosus) | PCR, culture | 0.05% (95% CI, 0.1–0.009) | Sanbonmatsu et al., 200540 |
| Madrid | 963 P. perniciosus | Culture | 0.21% (95% CI, 0.06–0.75) | Remoli et al., 201647 | |
| Granada | Granada and Ibiza | 108 batches of 50–100 sandflies (42 females) | PCR, culture | 12 positive batches | Collao et al., 201048 |
| Barcelona, Granada and Ibiza | 307 batches of sandflies | PCR | 25 positive batches | Sanchez-Seco et al., 201049 | |
| Arbia | Madrid | 366 P. perniciosus | Culture | 0.82% (95% CI, 0.28–2.3) | Remoli et al., 201647 |
| Arrabida-like | Madrid | 597 P. perniciosus | Culture | 0.17% (95% CI, 0.03–0.94) | Remoli et al., 201647 |
The first description of the presence of SBP in Spain was made by Eitrem in 1991, with the publication of a clinical case of Toscana virus infection in a Swedish tourist who had visited Catalonia.32 Since then, the virus has been detected in sporadic cases of meningitis or meningoencephalitis in residents of communities in Catalonia and Murcia, as well as in travellers who had visited Andalusia and the Mediterranean coast.33–37 Similarly, retrospective studies have been carried out on CSF or sera from people who had had aseptic meningitis, in which no aetiological diagnosis had been reached. Out of 1393 cases studied in different places, using different techniques, 58 (4.2%, 95% CI, 3.1–5.2) were positive (Table 2). Positive cases were more frequent in people from rural areas, with onset of symptoms between June and October.38Additionally, some serious complications of the disease have been described in the cases detected in our country, such as hearing loss, paresis or aphasia.39,40
The presence of the Toscana virus in Spain has also been demonstrated by antibody detection studies. The mean prevalence of seropositive participants varies, according to the studies, between 5% and 26%, with regional differences. The first study, conducted between 1988 and 1996, using IFI techniques found that the areas with the highest seroprevalence were Palma de Mallorca (61%), Jerez (31%), Barcelona (28%) and Granada (27%), unlike northern towns, such as Santiago de Compostela (11%) and San Sebastian (11%), which had the lowest prevalence levels.8 These observations have been corroborated in part in subsequent studies, where prevalence exceeded 25% of the general population in Granada and Mallorca, in contrast with Madrid and Catalonia, where it did not exceed 5%.34,40–42 In Catalonia and Granada, an age-dependent relationship has been observed, with higher seroprevalence in adults and the elderly.34,40
Similarly, significant exposure to the Toscana virus has been reported in domestic animals. In the province of Granada, seropositivity was found in sera from all species of domestic animals studied by IFI techniques. The highest seroprevalence rates were found in horses (64%), cats (60%) and dogs (48%). Antibodies were also detected in sheep (33%), pigs (22%), goats (18%) and cows (18%).45 Meanwhile, the presence of the Toscana virus in the vector has been demonstrated. In 2003, in studies carried out by the Enfermedades Víricas Transmitidas por Artrópodos y Roedores (EVITAR) [Viral Diseases Transmitted by Arthropods and Rodents] network, the presence of Toscana genetic material was demonstrated in 0.05% of the sandflies captured in the province of Granada.40 This prevalence, clearly lower than that identified in endemic areas of Italy, where the virus is detected in 0.2% of vectors, has led to a partial explanation of the lower incidence of the disease for years in southern Spain, compared to central Italy.40,46 Recently, in the municipality of Madrid, Toscana virus has been detected in 0.21% of P. perniciosus, similar to that found in the Tuscany region of Italy.40,47
Granada virusThe Granada virus has been isolated in sandflies in southern Spain, with no human cases having been confirmed to date. The complete analysis of its genome indicates that the Granada virus could be the result of genetic recombination of the Massilia virus, which would contribute the L and S segments, and another unidentified phlebovirus that would contribute the M segment.48 Several human seroprevalence studies have been performed using direct immunofluorescence techniques, showing a prevalence of Granada virus antibodies of 15% in the population of the province of Granada, although this technique can produce cross-reactions with the Toscana virus.6,48 By viral neutralisation, the estimation of the seroprevalence of this virus in two studies in Granada was 2% and 2.8%.6,48 In a study carried out on sandflies captured in Granada and Ibiza between 2004 and 2005, the Granada virus was detected by PCR and sequencing in 11% of the batches studied.48
Naples, Sicily, Arbia and Arrabida-like virusesThe Naples and Sicily viruses have not been detected in Spain or in humans, animals or sandflies. Antibodies against these or other related viruses have been detected in a study carried out in donor sera obtained between 1988 and 1996,8 but it is considered that the specificity of the signal obtained in this study should be analysed in greater depth, based on current knowledge.
The Arbia and Arrabida-like viruses have recently been isolated in Spain from infected sandflies in the municipality of Fuenlabrada in Madrid (Table 3).47 At the moment, the implication that this finding may have for human health is unknown.
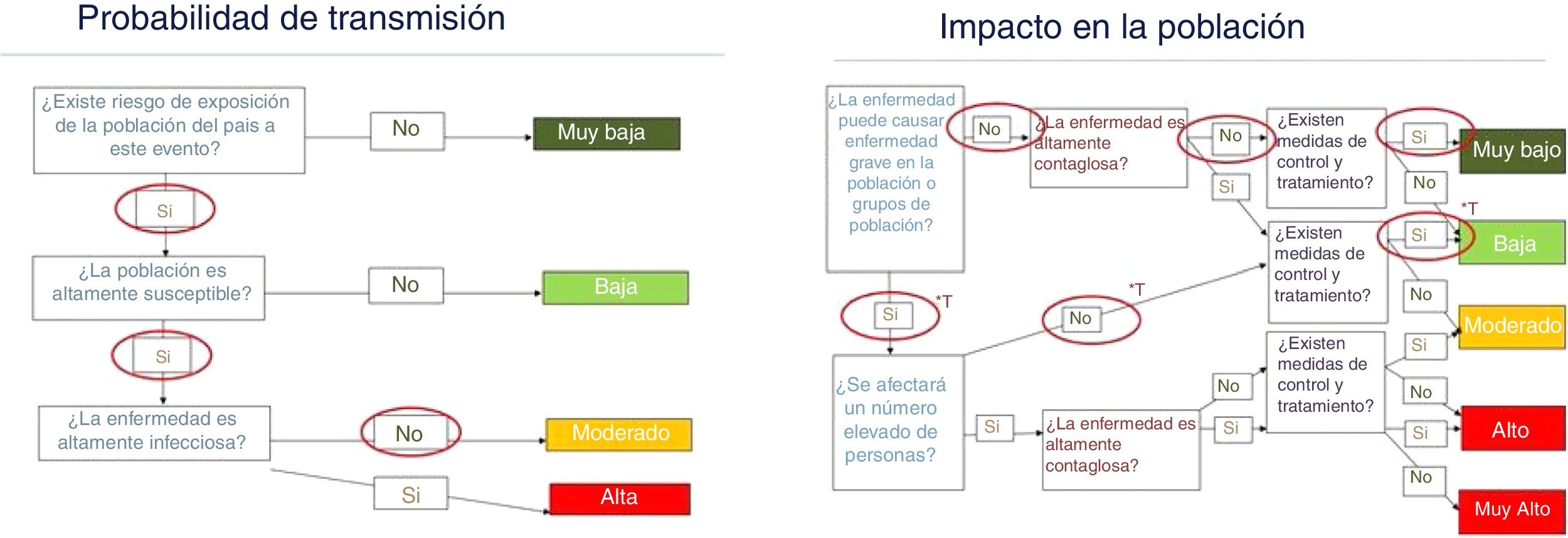
Risk assessment for SpainThe risk to public health of infectious diseases is predetermined by the risk of transmission and the impact that the disease causes in the population. The methodology used for this assessment is based on the best available evidence, as well as the qualitative evaluations of experts in the fields of infectology, epidemiology, microbiology, entomology and public health, using the methodology proposed by the European Centre for Disease Prevention and Control.50 The findings of this assessment serve to guide response preparedness plans and public health actions at the national level.
The factors that contribute to the urgency and scope of SBPs are complex and are related, among others, to characteristics of the viruses themselves, the favourable environmental conditions for the vector, and the factors that favour the contact of the vectors with the human being. SBPs have a high mutation rate, leading to the rapid generation of new viral serotypes. This could condition the appearance of new viruses or variants that are more transmissible or more pathogenic for humans. On the other hand, there is the possibility of the introduction of new SBPs through viraemic travellers from other continents.51 It is foreseeable that the generalised increase in temperatures that the planet is experiencing, together with changes in land use, will favour sandfly colonisation in more northern regions and also areas with higher altitude.52 Demographic and environmental changes may favour contact between vectors and humans. The rapid transformation of rural and agricultural areas into urban areas, and the consequent abandonment of agricultural practices, have given way to ecologically degraded areas where the vector could proliferate. The enormous urban development in Spain and, specifically, the increase in the number of single-family houses with gardens, which has taken place mainly around large cities,53 as well as the modification of large cities to create large protected green spaces, increase favourable conditions for the sandfly to develop in the proximity of human habitats. In general, the Spanish population would be susceptible to SBP infection, although a small percentage could be protected by the presence of antibodies generated by a previous exposure. The population with the highest risk of infection would obviously be the one that resides in areas where the vector is more abundant, especially at times of the year when densities are higher and the vector is more active.
SBP infections are generally asymptomatic and sometimes cause a self-limited febrile syndrome with flu-like symptoms. If we take into account the severity of the disease to measure the impact, only the Toscana virus can generate serious symptoms: meningoencephalitis, in general self-limited, although not exempt from occasional complications. However, there are reasons to believe that SBP infections, and especially those caused by the Toscana virus, are underdiagnosed. According to the different authors, the aetiology of aseptic meningitis would remain without a clear diagnosis in 40–60% of cases, and can be up to 81% depending on the series.54–57 In Spain, it is estimated that the Toscana virus would be the cause of 2.3–8% of aseptic meningitis cases that remain undiagnosed, according to retrospective studies carried out in the research context. This scenario may be due in part to the low degree of suspicion among clinicians, but also to the fact that the diagnostic test is not available in most health centres, nor is it included in the diagnostic panels for aseptic meningitis.
According to the available data, the risk of SBP transmission is moderate in the hot and dry areas of the country, and the Mediterranean coast in the summer and autumn months, and low in the rest of the country and in the cold months. The probability of symptomatic Toscana virus cases being detected would be moderate and the appearance of outbreaks in places where sandfly densities are higher cannot be ruled out. For the rest of the SBPs, this probability is very low, since most of the cases would be asymptomatic. The absence of specific diagnostic tests for some of these viruses, together with the low clinical suspicion among healthcare professionals at the present time, contribute to the fact that a large percentage of cases are not detected, thus making it difficult to take control measures. This, in turn, could increase the likelihood of transmission. Although some cases of Toscana virus disease can be serious, most are not, so the overall impact of the disease for the Spanish population is considered low for the Toscana virus and very low for the rest of the SBPs (Fig. 1).
Risk assessment for public health regarding phleboviruses transmitted by sandflies in territories and climatic conditions favourable for the development and activity of the vector.
Source: own development based on the risk assessment map of the European Centre for Disease Prevention and Control.
*T: estimate made only for the Toscana virus.
It seems to be necessary to increase scientific knowledge about SBPs in Spain, and so it would be important to include these viruses in the diagnostic schemes for aseptic meningitis in microbiology laboratories, at least in areas where there is greater risk. For this, it would be necessary to have a specific diagnostic methodology to differentiate the different SBPs, developing new methods at the molecular level and using the virus neutralisation technique, due to the high degree of cross-reactivity existing at the serological level. In order to control vector density, it would be important to develop strategies within the different public health authorities with the capacity to carry out environmental actions to modify the vector's habitat and apply appropriate insecticides, but always selectively. There currently exists a Plan Nacional de Preparación y Respuesta frente a Enfermedades Transmitidas por Vectores [National Plan for Preparedness and Response to Vector-Borne Diseases], which must be expanded to include the control of sandflies.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: García San Miguel L, Sierra MJ, Vazquez A, Fernandez-Martínez B, Molina R, Sanchez-Seco MP, et al. Enfermedades asociadas a flebovirus trasmitidos por flebótomos: ¿qué riesgo tenemos en España? Enferm Infecc Microbiol Clin. 2021;39:345–351.