Methicillin-resistant Staphylococcus aureus (MRSA) is a leading cause of hospital-acquired and healthcare-associated infections worldwide.1 MRSA bacteremia is associated with higher mortality than methicillin-susceptible S. aureus bacteremia.2 For MRSA bacteremia, the IDSA guidelines recommend treatment with vancomycin or daptomycin.3 However, strains showing higher vancomycin MICs have been associated with increased risk of treatment failure and death,4 and the emergence of non-susceptible mutants with daptomycin treatment is concerning.5 In view of these therapeutic difficulties, one possibility is to search for adjuvant substances that allow the continued use of conventional antimicrobials. Some studies have found that statins treatment has a protective effect against both mortality and the development of persistent bacteremia in patients with S. aureus bacteremia.6,7 Statins have no intrinsic bactericidal activity against S. aureus,8 but synergize with different antimicrobials to increase their bactericidal effect.9 In addition, it has been shown that several statins can reverse methicillin resistance in the community-associated MRSA strain USA300.8 Despite this phenomenon was confirmed in an experimental murine model,8 significant questions remain unresolved, and no studies have evaluated the impact of statins as a strategy to reverse methicillin resistance against clinical isolates of MRSA under human therapeutic concentrations. We tested statins against a set of clinical isolates of MRSA belonging to the most prevalent high-risk clones in human infections to address this question.
Nineteen MRSA isolates recovered from patients with nosocomial or healthcare-associated bacteremia were selected for this study. Isolates belonged to the following high-risk clones: ST5 (thirteen isolates), ST8 (four isolates), ST22 (one isolate) and ST45 (one isolate). Thirteen isolates belonged to agr group II and the remainder to agr group I. All isolates harboured SCCmec type IV, and none of the isolates carried Panton-Valentine leukocidin genes.10S. aureus ATCC 29213 and MRSA USA300 strains were included as controls.
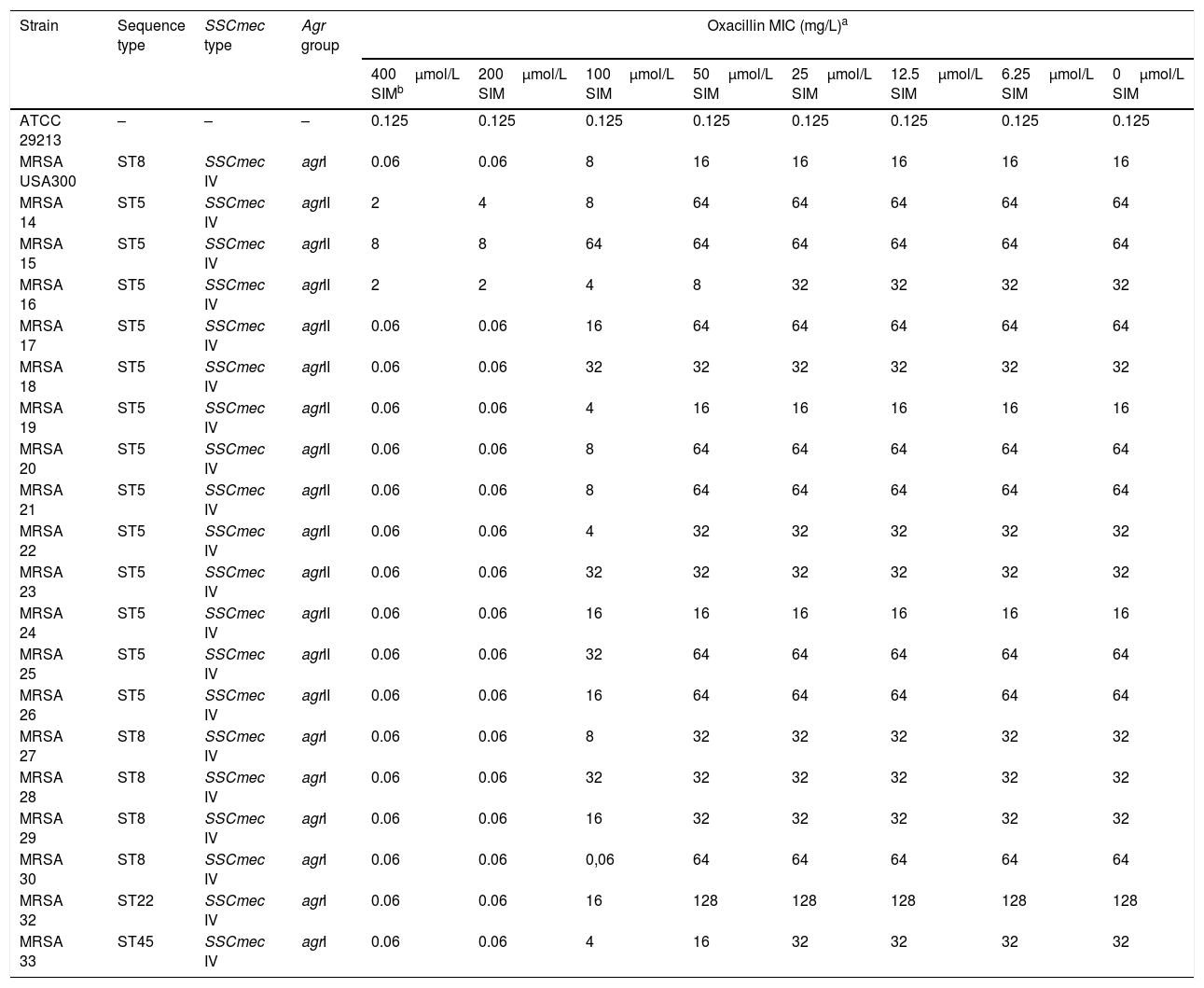
The active forms of five statins were tested: fluvastatin, rosuvastatin, atorvastatin, simvastatin and lovastatin (Axxora, Brussels, Belgium). In the case of simvastatin and lovastatin, the prodrug form (lactone) was also included (Axxora). Susceptibility to statins and oxacillin (Sigma-Aldrich, Madrid, Spain) was determined by broth microdilution. All isolates showed MIC values of all statins tested above 200μmol/L and oxacillin MICs≥16mg/L (Table 1). Statin–oxacillin synergy was evaluated using the chequerboard method. None of the statins evaluated showed synergistic effects with oxacillin, except for the prodrug form of simvastatin. The lactone form of simvastatin reversed methicillin resistance (defined as the reduction of oxacillin MIC to ≤2mg/L) in eighteen isolates (95%) at 400μmol/L, and in seventeen isolates (89%) at 200μmol/L. However, methicillin resistance reversion was not observed in any isolate at lower concentrations of simvastatin (Table 1). The MIC of the lactone form of simvastatin was >800μmol/L, which means that this effect is observed at sub-MIC concentrations of simvastatin.
Oxacillin MIC of MRSA clinical isolates in the presence of the lactone form of simvastatin.
| Strain | Sequence type | SSCmec type | Agr group | Oxacillin MIC (mg/L)a | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 400μmol/L SIMb | 200μmol/L SIM | 100μmol/L SIM | 50μmol/L SIM | 25μmol/L SIM | 12.5μmol/L SIM | 6.25μmol/L SIM | 0μmol/L SIM | ||||
| ATCC 29213 | – | – | – | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 |
| MRSA USA300 | ST8 | SSCmec IV | agrI | 0.06 | 0.06 | 8 | 16 | 16 | 16 | 16 | 16 |
| MRSA 14 | ST5 | SSCmec IV | agrII | 2 | 4 | 8 | 64 | 64 | 64 | 64 | 64 |
| MRSA 15 | ST5 | SSCmec IV | agrII | 8 | 8 | 64 | 64 | 64 | 64 | 64 | 64 |
| MRSA 16 | ST5 | SSCmec IV | agrII | 2 | 2 | 4 | 8 | 32 | 32 | 32 | 32 |
| MRSA 17 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 16 | 64 | 64 | 64 | 64 | 64 |
| MRSA 18 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 32 | 32 | 32 | 32 | 32 | 32 |
| MRSA 19 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 4 | 16 | 16 | 16 | 16 | 16 |
| MRSA 20 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 8 | 64 | 64 | 64 | 64 | 64 |
| MRSA 21 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 8 | 64 | 64 | 64 | 64 | 64 |
| MRSA 22 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 4 | 32 | 32 | 32 | 32 | 32 |
| MRSA 23 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 32 | 32 | 32 | 32 | 32 | 32 |
| MRSA 24 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 16 | 16 | 16 | 16 | 16 | 16 |
| MRSA 25 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 32 | 64 | 64 | 64 | 64 | 64 |
| MRSA 26 | ST5 | SSCmec IV | agrII | 0.06 | 0.06 | 16 | 64 | 64 | 64 | 64 | 64 |
| MRSA 27 | ST8 | SSCmec IV | agrI | 0.06 | 0.06 | 8 | 32 | 32 | 32 | 32 | 32 |
| MRSA 28 | ST8 | SSCmec IV | agrI | 0.06 | 0.06 | 32 | 32 | 32 | 32 | 32 | 32 |
| MRSA 29 | ST8 | SSCmec IV | agrI | 0.06 | 0.06 | 16 | 32 | 32 | 32 | 32 | 32 |
| MRSA 30 | ST8 | SSCmec IV | agrI | 0.06 | 0.06 | 0,06 | 64 | 64 | 64 | 64 | 64 |
| MRSA 32 | ST22 | SSCmec IV | agrI | 0.06 | 0.06 | 16 | 128 | 128 | 128 | 128 | 128 |
| MRSA 33 | ST45 | SSCmec IV | agrI | 0.06 | 0.06 | 4 | 16 | 32 | 32 | 32 | 32 |
Several studies have shown the pleiotropic effects of statins, including bactericidal activity against gram-positive bacteria, such as S. pneumoniae, and reversion of methicillin resistance in S. aureus.8,11 These effects were detected at statin concentrations of 1–100μmol/L. Nevertheless, the mean concentration of statins in human serum is only 1–15nmol/L, and the peak concentration is 6–50nmol/L, with this concentration being reached in serum for a very short period of time.12
According to our results, the inactive form of simvastatin is the only statin tested which is able to reverse methicillin resistance in S. aureus clinical isolates, although this effect was observed at sub-MIC simvastatin concentrations above 100μmol/L. Moreover, this concentration of statin is much higher than that reached in serum with therapeutic doses, so that it cannot be used as a potential adjuvant to reverse methicillin resistance. Why this effect is not observed with the prodrug form of simvastatin remains unknown.
These results contrast with those previously found in a murine model of pulmonary infection.8 This discrepancy might be partially explained by the differences in dosage. In humans, the dosage of statins is approximately 0.1–1mg/kg, while in the murine model, doses of 20 and 50mg/kg were used. However, these doses were similar to those used in previous in vitro studies.11 The metabolism, pharmacodynamics and action of statins differ in humans and mice so that the results observed in murine models should be taken with caution before being applied to humans.
This study shows that simvastatin maybe a potential strategy to reverse methicillin resistance in S. aureus, although, unfortunately, this effect is not observed at therapeutic human concentrations.
FundingThis work was supported by the Plan Nacional de I+D+i 2017–2020 and the Instituto de Salud Carlos III (project PI18/00715), Subdirección General de Redes y Centros de InvestigaciónCooperativa, Ministerio de Economía, Industria y Competitividad, the Spanish Network for Research in Infectious Diseases (REIPI; RD16/0016/0001 and REIPI RD16/0016/0009), co-financed by the European Development Regional Fund“A Way to Achieve Europe,” operative programme Intelligent Growth 2014–2020.
Conflicts of interestNone to declare.
The authors would like to thank Dr. Carmen Velasco for giving us the S. aureus clinical isolates used in this study.