To analyse and compare predictive factors of bacterial meningitis in the patients seen in the Emergency Departments (ED) due to an episode of acute meningitis (AM).
MethodsA prospective, observational study was carried out in patients aged 15 years and older seen in ED due to AM between August 2009 and November 2015. Thirty-two variables for predicting bacterial meningitis were assessed. They covered epidemiological, comorbidity, clinical and analytical factors. Multivariate logistic regression analysis was performed.
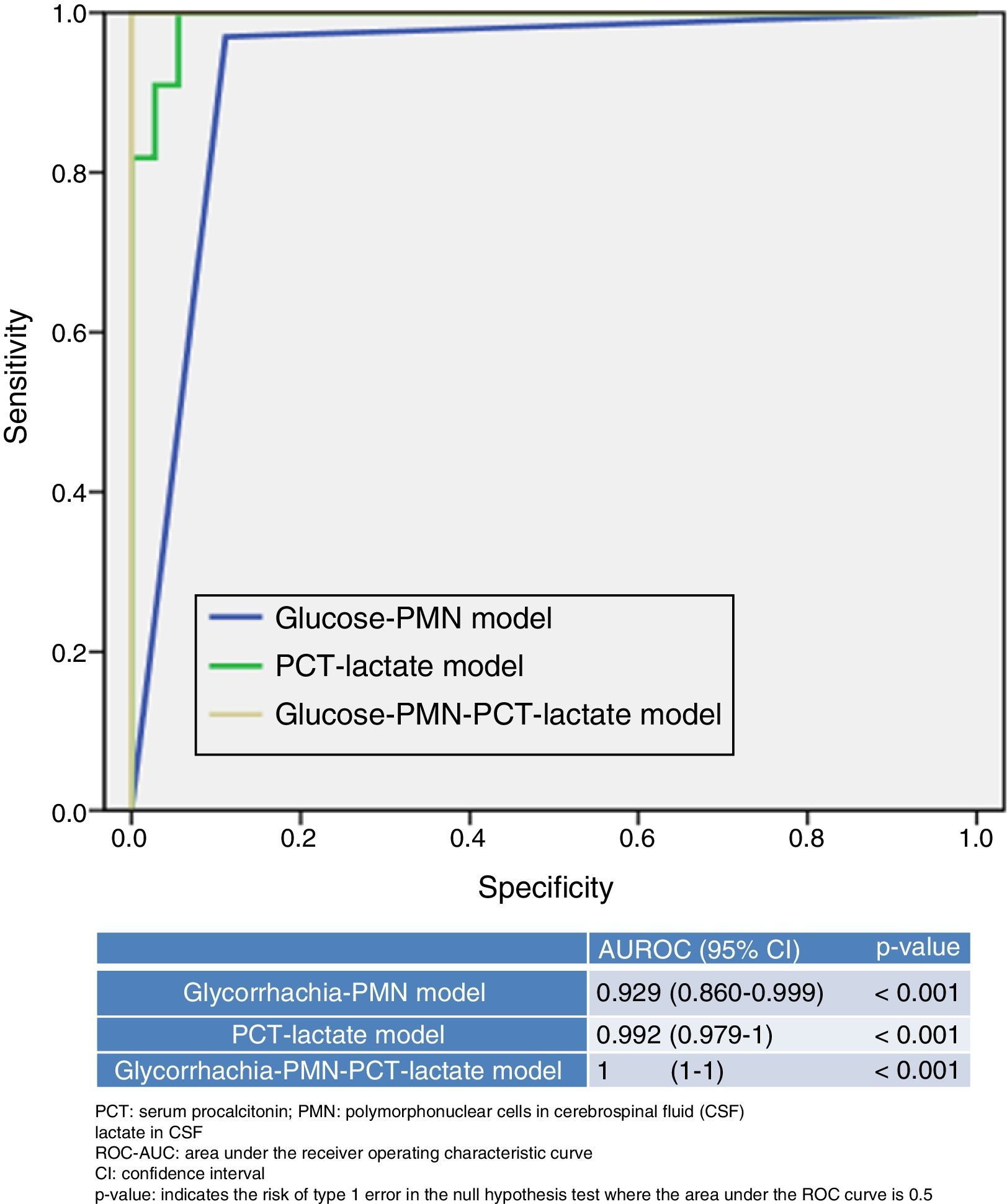
ResultsThe study included 154 patients. The diagnosis was bacterial meningitis in 53 (34.4%) patients. Four variables were significantly associated with bacterial aetiology: cerebrospinal fluid (CSF) lactate concentration ≥33mg/dl (odds ratio [OR] 50.84; 95% confidence interval [CI]: 21.63–119.47, p<.001), serum procalcitonin (PCT) ≥0.8ng/ml (OR 46.34; 95% CI: 19.71–108.89; p<.001), CSF glucose <60% of blood value (OR 20.82; 95% CI: 8.86–48.96; p=.001), CSF polymorphonuclears greater than 50% (OR 20.19; 95% CI: 8.31–49.09; p=.002]. The area under the curve for the model serum PCT≥0.8ng/ml plus CSF lactate ≥33mg/dl was 0.992 (95% CI: 0.979–1; p<.001), and achieved 99% sensitivity and 98% specificity for predicting bacterial meningitis.
ConclusionsSerum PCT with CSF lactate, CSF glucose and CSF polymorphonuclears evaluated in an initial assessment in the ED for patients with AM, achieved an excellent diagnostic usefulness for predicting bacterial meningitis.
Analizar y comparar los factores predictivos de meningitis bacteriana (MB) en los pacientes que acuden al servicio de urgencias (SU) por un episodio de meningitis aguda (MA).
MétodosEstudio observacional y prospectivo de pacientes adultos (≥15años) diagnosticados de MA en un SU desde agosto de 2009 hasta noviembre de 2015. Se analizaron 32 variables (epidemiológicas, de comorbilidad, clínicas y analíticas) que pudieran predecir etiología bacteriana. Se realizó un estudio multivariable mediante regresión logística.
ResultadosSe incluyó a 154 pacientes, de los que 53 (34,4%) fueron MB. Cuatro variables se asociaron de forma significativa como predictores de etiología bacteriana: lactato en líquido cefalorraquídeo (LCR) ≥33mg/dl (odds ratio [OR]: 50,84; intervalo de confianza [IC] al 95%: 21,63-119,47; p<0,001); procalcitonina (PCT) sérica ≥0,8ng/ml (OR: 46,34; IC95%: 19,71-108,89; p<0,001), glucorraquia <60% de la glucemia (OR: 20,82; IC95%: 8,86-48,96; p=0,001), proporción de polimorfonucleares (PMN) en LCR >50% (OR: 20,19; IC 95%: 8,31-49,09; p=0,002). El área bajo la curva-ROC del modelo (PCT≥0,8ng/ml+lactato en LCR ≥33mg/dl) fue 0,992 (IC95%: 0,979-1; p<0,001) y consigue una sensibilidad del 99% y una especificidad del 98% para predecir MB.
ConclusionesLa PCT sérica, junto con la valoración del lactato, glucorraquia y proporción de PMN del LCR en la evaluación inicial del paciente con sospecha de MA en el SU, tienen un excelente rendimiento diagnóstico para predecir la MB.
Bacterial meningitis (BM) refers to inflammation of the leptomeninges that tends to present classic signs in the patient's cerebrospinal fluid (CSF): intense pleocytosis with a predominance of polymorphonucleocytes (PMN) together with increased CSF protein levels and a fall in glycorrhachia (CSF sugar levels).1 Although it does not feature amongst the 10 most commonly-treated adult infections in emergency departments (EDs),2 it is more commonly associated with sepsis, severe sepsis and septic shock criteria than any other type of infection, which, together with its clinical significance, reflects its severity.2,3 Likewise, although it is not among the 10 most common causes of death in the ED in absolute terms,4 the complications and mortality that can present in the ED itself or in the first 24h of hospital admission continue to be high, despite its low incidence. In light of the above, suspecting bacterial infection in acute meningitis (AM) remains a challenge of significant importance in the ED, until cultures and microbiological tests can confirm its viral or bacterial origin.5 Priority of care for patients with suspected AM should be identified and implemented from the initial patient assessment or triage.6 The aim for emergency departments should be to predict a suspected BM diagnosis with as much certainty as possible using just the tools that tend to be readily available in EDs (medical history, physical examination and complementary tests),7 until microbiological tests confirm its true aetiology.
The medical history and clinical manifestations of BM do not differ from viral meningitis (VM).1,8 These manifestations tend to be more nonspecific in newborns and the elderly, as well as in immunosuppressed patients, diabetics and other patients who are particularly susceptible to severe infection, in whom the common signs and symptoms do not offer sufficient sensitivity or specificity to initially suspect AM or to subsequently distinguish between potential BM and VM.8–10
Furthermore, the appropriate and early administration of antibiotics is very important for a positive patient outcome and for survival.11,12 There is therefore an argument to be made to equip EDs with the appropriate and instant resources to be able to suspect and distinguish between BM and VM cases. These include inflammatory and infection biomarkers, and particularly procalcitonin (PCT) in blood tests due to its greater diagnostic performance.13–16 In terms of CSF analysis, recent publications have found lactate to have the highest predictive diagnostic value of BM, ahead of cell count (pleocytosis), the proportion of PMN or existing glycorrhachia.17,18
In light of the above, the primary objective of this study was to determine which identifiable factors from those used in the initial emergency department patient assessment, including medical history and clinical examination, determination of biomarkers in blood and CSF analysis, correlate with a microbiological diagnosis of bacterial meningitis in patients with suspected AM. The secondary objective was to assess the individual and combined diagnostic performance of the factors found to have the highest diagnostic value.
Patients and methodDesignA prospective, observational study of patients diagnosed with AM in the ED was conducted. Patients were followed-up until death in the hospital or for 30 days following admission to the ED.
Study siteThe study was conducted at Complejo Hospitalario de Toledo, a tertiary hospital with 786 beds and a catchment area of 437,000 people. An average of 448 emergencies/day were treated during the study period.
Study periods and population studiedAll patients aged 15 years and above diagnosed with AM at the ED and who were subjected to a lumbar puncture were consecutively enrolled between 15 August 2009 and 15 November 2015. Infection biomarkers (C-reactive protein [CRP] and PCT) and the other analytical parameters that form part of the study were analysed from patient blood cultures and blood test samples.
Based on the final confirmed diagnosis, the patient sample was split into 6 groups:
- 1.
BM before isolating the pathogen or its capsular antigens in the CSF (in this case, the patient was also checked for concomitant bacteraemia).
- 2.
VM when confirmed by polymerase chain reaction (PCR) for herpesviruses (DNA amplification) and enteroviruses (RNA amplification) in the CSF.
- 3.
Probable VM, with a negative bacterial culture both in the CSF and in the blood cultures.
- 4.
Possible partially-treated BM when a bacterial profile was identified in the CSF coupled with antibiotic use in the previous 72h and negative CSF cultures and blood cultures.
- 5.
Possible AM with bacterial profile, with no prior use of antibiotics and with negative CSF cultures and blood cultures.
- 6.
AM with microbiological confirmation following tuberculous meningitis.
Patients with a suspected primary bacterial infection not related to the AM episode (except pharyngitis, sinusitis or otitis) were excluded to prevent potential biomarker false positives,14 as were patients diagnosed with a second case of AM during the study period or autoimmune meningitis during follow-up, patients with neurosurgical history and patients with intraventricular device infections and cerebrospinal fluid shunt infections.
The study met the ethical standards of our hospital. All encrypted data were treated confidentially and were not disseminated beyond the team of investigators. The follow-up of all patients was performed by means of the hospital's computerised medical records and by primary care. The study did not involve any therapeutic procedure or have any clinical implications.
Recorded variablesThe following variables were recorded: sociodemographic variables (age, gender) and comorbidities (existence of a solid tumour disease, oncological disease or haematological disease, liver disease, nephropathy, diabetes, chronic or cerebrovascular heart disease), human immunodeficiency virus (HIV) infection, immunosuppression (patients with solid organ transplant or splenectomy, treated with 10mg or more of prednisone per day or equivalent for more than 30 days, or treated with other immunosuppressants in the last year). The Charlson index was calculated.19 Clinical variables were collected (fever with a temperature ≥38°C, altered level of consciousness, headache, stiff neck and signs of meningeal irritation [Kernig's or Brudzinski's sign] and exanthema) as were the severity variables listed in the systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock criteria according to the 2001 International Sepsis Definitions Conference.20 The use of an antibiotic in the 72h prior to visiting the ED, the time since symptom onset, length of hospital stay and mortality (until the death of the patient in the hospital or for 30 days following admission) were also added to the list of variables.
The following analytical variables were analysed from all patients: complete blood count, coagulation test, basic clinical chemistry, serum CRP and PCT concentrations, CSF characteristics (pleocytosis and proportion of PMN, protein levels, glycorrhachia and its proportion in relation to simultaneous blood glucose levels and lactate concentration). The reference values chosen for the tests, and the dichotomisations, were arbitrarily defined by the authors. This was done by referring to the values defined as normal by our laboratory (shown below), which were also used in some recently-published studies that have been taken as an example.14,16–18 The microbiological tests performed were: blood cultures; Gram and Ziehl-Neelsen staining; CSF culture; detection of capsular antigens; and polymerase chain reaction (PCR) detection of DNA (herpesviruses) and RNA (enteroviruses) by amplification.
Definitions, techniques and methods established for the samplesThe normal reference values of our laboratory were adopted: CRP: 0–8mg/l and PCT<0.5ng/ml. CRP was determined by quantitative ELISA (Vitros CRP® Slides) with a sensitivity of 1mg/l, while PCT was determined by quantitative electrochemiluminescence immunoassay (Elecsys Brahms PCT®) with a sensitivity of 0.02ng/ml.
The CSF test was considered normal (according to the reference values of our laboratory) with pleocytosis fewer than 10leukocytes/mm3, glycorrhachia between 60 and 80% of serum glucose, CSF protein levels of 15–45mg/dl and lactate <35mg/dl.16–18 As mentioned above, some variables were arbitrarily dichotomised14,16–18: CSF lactate concentration dichotomised when >35mg/dl; blood leucocyte count (mm3) dichotomised when >12,000 or <4000 or >10% of band cells; CRP concentration (mg/l) after dichotomising if >8mg/l and if >54mg/l, and serum PCT concentration (ng/ml) after dichotomising when >0.5 and if >1ng/ml.
Pathological CSF was defined as: (1) a bacterial profile (increased cell count with a predominance of PMN, increased CSF protein levels and decreased glycorrhachia); (2) a lymphocytic profile (with a predominance of mononuclear cells with normal or decreased glycorrhachia); and (3) an inconclusive profile (when the aforementioned criteria were not met). Blood cultures: 2 aerobic vials and 2 anaerobic vials (BD Bactec®) processed using the automated Bactec/Alert® system (BioMérieux, Durham, NC, USA).
Statistical analysisFor the statistical analysis, means and their standard deviations (SD) were used for quantitative variables, and percentages for qualitative variables. The chi-square test, Fisher's exact test, Student's t-test and the Mann–Whitney U test were used, as applicable, to investigate the correlation between the BM diagnosis and the independent variables (demographic characteristics and comorbidities, clinical variables, severity variables, progression variables, analytical variables and dichotomised variables).
A multivariate logistic regression analysis was conducted (the “enter method” was chosen to include all the chosen variables of the model, that is, all those that had statistical significance in the univariate analysis). The comparison results were expressed as p-values and odds ratios (OR) with a 95% confidence interval (95% CI). A p-value <0.05 or when the 95% CI of the OR excluded the value 1 was accepted as significant.
The efficacy of the BM predictive capacity was assessed by analysing ROC (receiver operating characteristic) curves with a 95% CI of the area under the ROC curve (AUC), which was compared against the neutral value (0.5). The standard errors of the AUC were calculated by non-parametric methods.
For the variables that yielded the highest diagnostic performance (ROC-AUC) in serum (PCT) and CSF (lactate), Youden's index was used to determine the cut-off points with the highest diagnostic value that maximised the difference between the true positive rate and the false positive rate. The sensitivity (SEN), specificity (SPE), positive predictive value (PPV), negative predictive value (NPV), the positive likelihood ratio (LR+) and the negative likelihood ratio (LR−) of the chosen cut-off points for the results studied were found. Their 95% CI were found by exact binomial methods and by the Taylor series method in the case of the likelihood ratios.
Adjusted logistic regression differentiated for each independent variable was used to study the BM predictive capacity of the combinations studied (serum PCT and CSF lactate, CSF PMN and glycorrhachia, and the combination of the sum of these 2 models). The likelihoods calculated as detailed above were subjected to a ROC curve analysis and the same procedures as for the individual markers.
A p-value <0.05 was considered to be significant and all the tests were two-tailed. The programme IMB-SPSS® Statistics 19 for Windows was used.
Results154 patients who met the inclusion and follow-up criteria (maintaining the AM diagnosis established by the ED at 30 days) were enrolled during the study period. The mean age (standard deviation [SD]) was 44 (22) years and 59.7% were male (92 cases).
Table 1 shows the final microbiological diagnosis of all patients enrolled in the study. They comprised 53 BM diagnoses confirmed by CSF culture and/or blood culture, 35 cases of VM, 42 classified as “probable VM” with negative cultures (CSF and blood cultures) and a CSF lymphocyte profile, 12 possible partially-treated bacterial AM (with a history of antibiotic use and negative cultures), 10 AM with no microbiological diagnosis but with a CSF bacterial profile and 2 cases of confirmed tuberculous meningitis. 32 patients with BM (60.4%) had associated bacteraemia.
Definitive microbiological diagnosis.
| Microbiological diagnosis | n=154 |
|---|---|
| Bacterial meningitis | 53 |
| Streptococcus pneumoniae | 27 (17 with BC+) |
| Neisseria meningitidis | 9 (6 with BC+) |
| Listeria monocytogenes | 7 (4 with BC+) |
| Haemophilus influenzae | 4 (1 with BC+) |
| Escherichia coli | 4 (2 with BC+) |
| Streptococcus milleri | 1 (1 with BC+) |
| Proteus mirabilis | 1 (1 with BC+) |
| CSF-confirmed viral meningitisa | 35 |
| Enterovirus | 32 |
| Herpes simplex virus | 3 |
| Probable viral meningitisb | 42 |
| Possible partially-treated bacterial meningitisc | 12 |
| Meningitis with no microbiological diagnosis with bacterial or inconclusive profiled | 10 |
| Mycobacterium tuberculosise | 2 |
BC+: positive blood cultures; CSF: cerebrospinal fluid; n: number of cases.
Viral microbiological diagnosis established by RNA amplification (polymerase chain reaction) for enterovirus and DNA amplification for herpes simplex virus.
CSF bacterial profile with history of antibiotic use in the previous 72h with negative CSF culture and negative blood cultures.
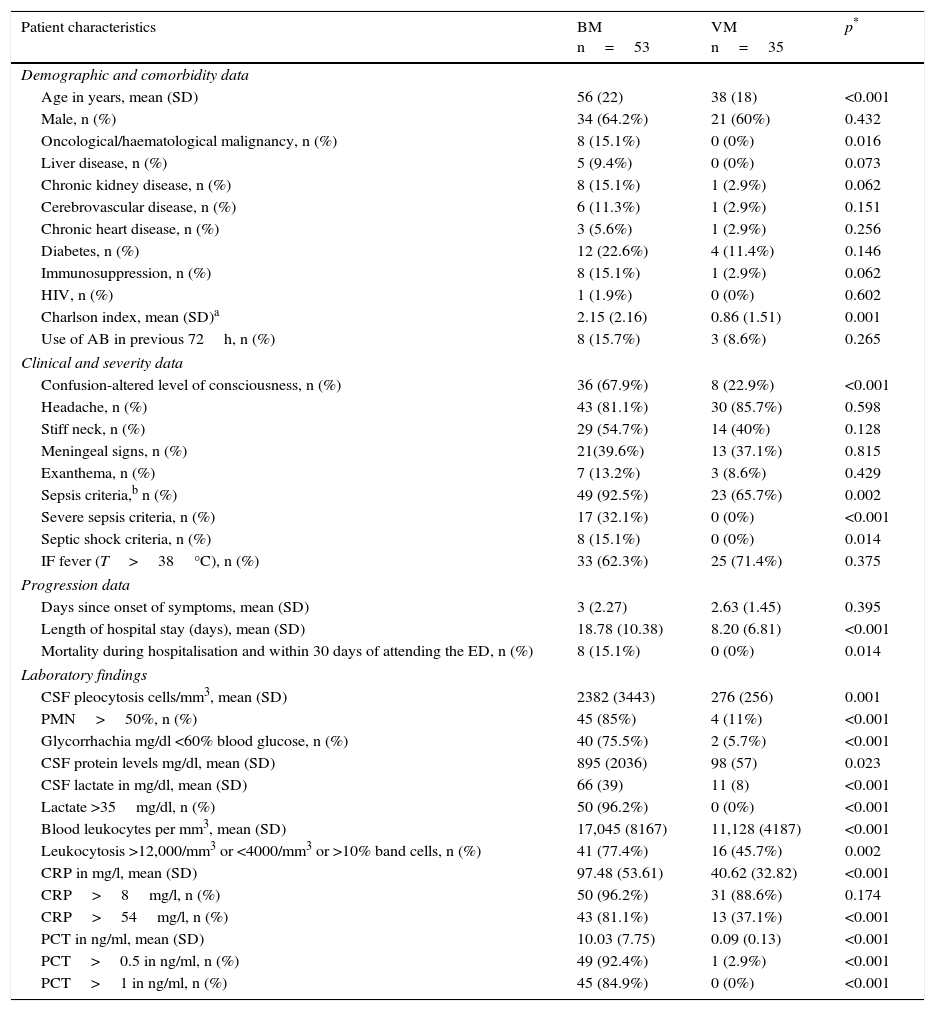
Table 2 shows the sociodemographic characteristics, comorbidities, Charlson index calculation, clinical data, severity and progression variables, the results of the emergency department lab tests of patients who attended the ED with a final diagnosis of BM and those with a confirmed VM diagnosis, and the univariate analysis comparing the two groups. Significant differences between the 2 groups were found in terms of age, various comorbidities and the Charlson index. In terms of clinical presentation, more patients in the BM group experienced altered level of consciousness than in the VM group (67.9% vs 22.9%, p<0.001), but no differences were found in the onset of fever, headache, stiff neck, signs of meningeal irritation or exanthema between the confirmed BM group and the confirmed VM group. There were also no differences in the previous use of antimicrobials or in the time elapsed from symptom onset to attending the ED. However, significant differences were found in terms of the frequency of cases with sepsis criteria (p=0.002), severe sepsis and septic shock criteria (p<0.001), with higher prevalence in the BM group than the VM group. Mean length of hospital stay and 30-day mortality after hospital admission were also both higher in the BM group.
Clinical, epidemiological, analytical and progression characteristics of patients treated at the emergency department with a final diagnosis of confirmed bacterial meningitis or viral meningitis (univariate analysis).
| Patient characteristics | BM n=53 | VM n=35 | p* |
|---|---|---|---|
| Demographic and comorbidity data | |||
| Age in years, mean (SD) | 56 (22) | 38 (18) | <0.001 |
| Male, n (%) | 34 (64.2%) | 21 (60%) | 0.432 |
| Oncological/haematological malignancy, n (%) | 8 (15.1%) | 0 (0%) | 0.016 |
| Liver disease, n (%) | 5 (9.4%) | 0 (0%) | 0.073 |
| Chronic kidney disease, n (%) | 8 (15.1%) | 1 (2.9%) | 0.062 |
| Cerebrovascular disease, n (%) | 6 (11.3%) | 1 (2.9%) | 0.151 |
| Chronic heart disease, n (%) | 3 (5.6%) | 1 (2.9%) | 0.256 |
| Diabetes, n (%) | 12 (22.6%) | 4 (11.4%) | 0.146 |
| Immunosuppression, n (%) | 8 (15.1%) | 1 (2.9%) | 0.062 |
| HIV, n (%) | 1 (1.9%) | 0 (0%) | 0.602 |
| Charlson index, mean (SD)a | 2.15 (2.16) | 0.86 (1.51) | 0.001 |
| Use of AB in previous 72h, n (%) | 8 (15.7%) | 3 (8.6%) | 0.265 |
| Clinical and severity data | |||
| Confusion-altered level of consciousness, n (%) | 36 (67.9%) | 8 (22.9%) | <0.001 |
| Headache, n (%) | 43 (81.1%) | 30 (85.7%) | 0.598 |
| Stiff neck, n (%) | 29 (54.7%) | 14 (40%) | 0.128 |
| Meningeal signs, n (%) | 21(39.6%) | 13 (37.1%) | 0.815 |
| Exanthema, n (%) | 7 (13.2%) | 3 (8.6%) | 0.429 |
| Sepsis criteria,b n (%) | 49 (92.5%) | 23 (65.7%) | 0.002 |
| Severe sepsis criteria, n (%) | 17 (32.1%) | 0 (0%) | <0.001 |
| Septic shock criteria, n (%) | 8 (15.1%) | 0 (0%) | 0.014 |
| IF fever (T>38°C), n (%) | 33 (62.3%) | 25 (71.4%) | 0.375 |
| Progression data | |||
| Days since onset of symptoms, mean (SD) | 3 (2.27) | 2.63 (1.45) | 0.395 |
| Length of hospital stay (days), mean (SD) | 18.78 (10.38) | 8.20 (6.81) | <0.001 |
| Mortality during hospitalisation and within 30 days of attending the ED, n (%) | 8 (15.1%) | 0 (0%) | 0.014 |
| Laboratory findings | |||
| CSF pleocytosis cells/mm3, mean (SD) | 2382 (3443) | 276 (256) | 0.001 |
| PMN>50%, n (%) | 45 (85%) | 4 (11%) | <0.001 |
| Glycorrhachia mg/dl <60% blood glucose, n (%) | 40 (75.5%) | 2 (5.7%) | <0.001 |
| CSF protein levels mg/dl, mean (SD) | 895 (2036) | 98 (57) | 0.023 |
| CSF lactate in mg/dl, mean (SD) | 66 (39) | 11 (8) | <0.001 |
| Lactate >35mg/dl, n (%) | 50 (96.2%) | 0 (0%) | <0.001 |
| Blood leukocytes per mm3, mean (SD) | 17,045 (8167) | 11,128 (4187) | <0.001 |
| Leukocytosis >12,000/mm3 or <4000/mm3 or >10% band cells, n (%) | 41 (77.4%) | 16 (45.7%) | 0.002 |
| CRP in mg/l, mean (SD) | 97.48 (53.61) | 40.62 (32.82) | <0.001 |
| CRP>8mg/l, n (%) | 50 (96.2%) | 31 (88.6%) | 0.174 |
| CRP>54mg/l, n (%) | 43 (81.1%) | 13 (37.1%) | <0.001 |
| PCT in ng/ml, mean (SD) | 10.03 (7.75) | 0.09 (0.13) | <0.001 |
| PCT>0.5 in ng/ml, n (%) | 49 (92.4%) | 1 (2.9%) | <0.001 |
| PCT>1 in ng/ml, n (%) | 45 (84.9%) | 0 (0%) | <0.001 |
AB: antibiotic; AM: acute meningitis; BM: bacterial meningitis confirmed by microbiology; CRP: C-reactive protein; CSF: cerebrospinal fluid; ED: emergency department; HIV: human immunodeficiency virus; n: number of cases; PCT: procalcitonin; PMN: polymorphonucleocytes; SD: standard deviation; T: temperature.
In terms of the emergency department lab tests, the univariate analysis (Table 2) showed a direct correlation between BM and the following 8 variables: degree of pleocytosis (cells/mm3); proportion of PMN>50%; glycorrhachia (mg/dl)<60% simultaneous blood glucose; CSF protein levels (mg/dl); CSF lactate concentration (mg/dl) (and dichotomised when >35mg/dl); blood leucocyte count (mm3) (and dichotomised when >12,000 or <4000 or >10% band cells); CRP concentration (mg/l) (and after dichotomising if >54mg/l), and concentration of serum PCT (ng/ml) (and after dichotomising when >0.5 and >1ng/ml).
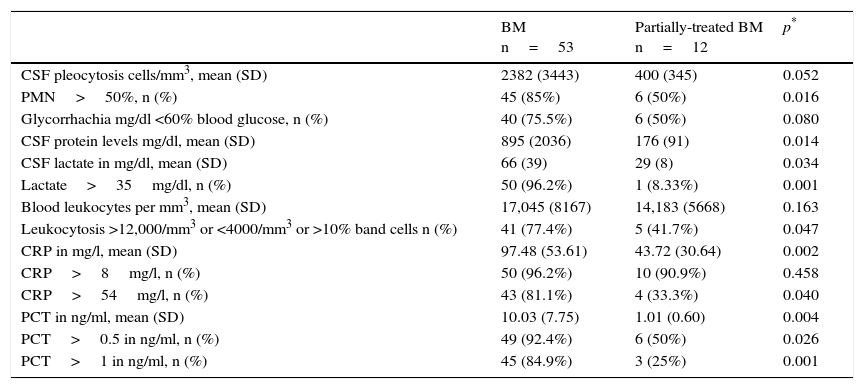
Similarly, Table 3 specifically compares the laboratory variables with confirmed cases of BM and possible cases of partially-treated BM. The table only reveals significant differences in the proportion of PMN >50%, CSF protein levels, CSF lactate concentration (and when >35mg/dl), serum CRP (and when >54mg/l) and PCT (both when >0.5 and >1ng/ml). However, all differences found were smaller than the differences between BM and VM (Table 2).
Comparison of the emergency department laboratory tests results in serum and CSF between patients with microbiologically-confirmed BM and possible partially-treated BM.
| BM n=53 | Partially-treated BM n=12 | p* | |
|---|---|---|---|
| CSF pleocytosis cells/mm3, mean (SD) | 2382 (3443) | 400 (345) | 0.052 |
| PMN>50%, n (%) | 45 (85%) | 6 (50%) | 0.016 |
| Glycorrhachia mg/dl <60% blood glucose, n (%) | 40 (75.5%) | 6 (50%) | 0.080 |
| CSF protein levels mg/dl, mean (SD) | 895 (2036) | 176 (91) | 0.014 |
| CSF lactate in mg/dl, mean (SD) | 66 (39) | 29 (8) | 0.034 |
| Lactate>35mg/dl, n (%) | 50 (96.2%) | 1 (8.33%) | 0.001 |
| Blood leukocytes per mm3, mean (SD) | 17,045 (8167) | 14,183 (5668) | 0.163 |
| Leukocytosis >12,000/mm3 or <4000/mm3 or >10% band cells n (%) | 41 (77.4%) | 5 (41.7%) | 0.047 |
| CRP in mg/l, mean (SD) | 97.48 (53.61) | 43.72 (30.64) | 0.002 |
| CRP>8mg/l, n (%) | 50 (96.2%) | 10 (90.9%) | 0.458 |
| CRP>54mg/l, n (%) | 43 (81.1%) | 4 (33.3%) | 0.040 |
| PCT in ng/ml, mean (SD) | 10.03 (7.75) | 1.01 (0.60) | 0.004 |
| PCT>0.5 in ng/ml, n (%) | 49 (92.4%) | 6 (50%) | 0.026 |
| PCT>1 in ng/ml, n (%) | 45 (84.9%) | 3 (25%) | 0.001 |
BM: bacterial meningitis; CRP: C-reactive protein; CSF: cerebrospinal fluid; n: number of cases; PCT: procalcitonin; PMN: polymorphonucleocytes; SD: standard deviation.
Although the univariate analysis (Table 2) found significant differences in numerous variables, only 4 of these maintained statistical significance as predictors of bacterial aetiology in AM in the multivariate regression analysis: CSF lactate ≥33mg/dl (OR: 50.84; 95% CI: 21.63–119.47; p<0.001); PCT ≥0.8ng/ml (OR: 46.34; 95% CI: 19.71–108.89; p<0.001); glycorrhachia <60% of blood glucose (OR: 20.82; 95% CI: 8.86–48.96; p=0.001] and the proportion of CSF PMN >50% (OR: 20.19; 95% CI: 8.31–49.09; p=0.002).
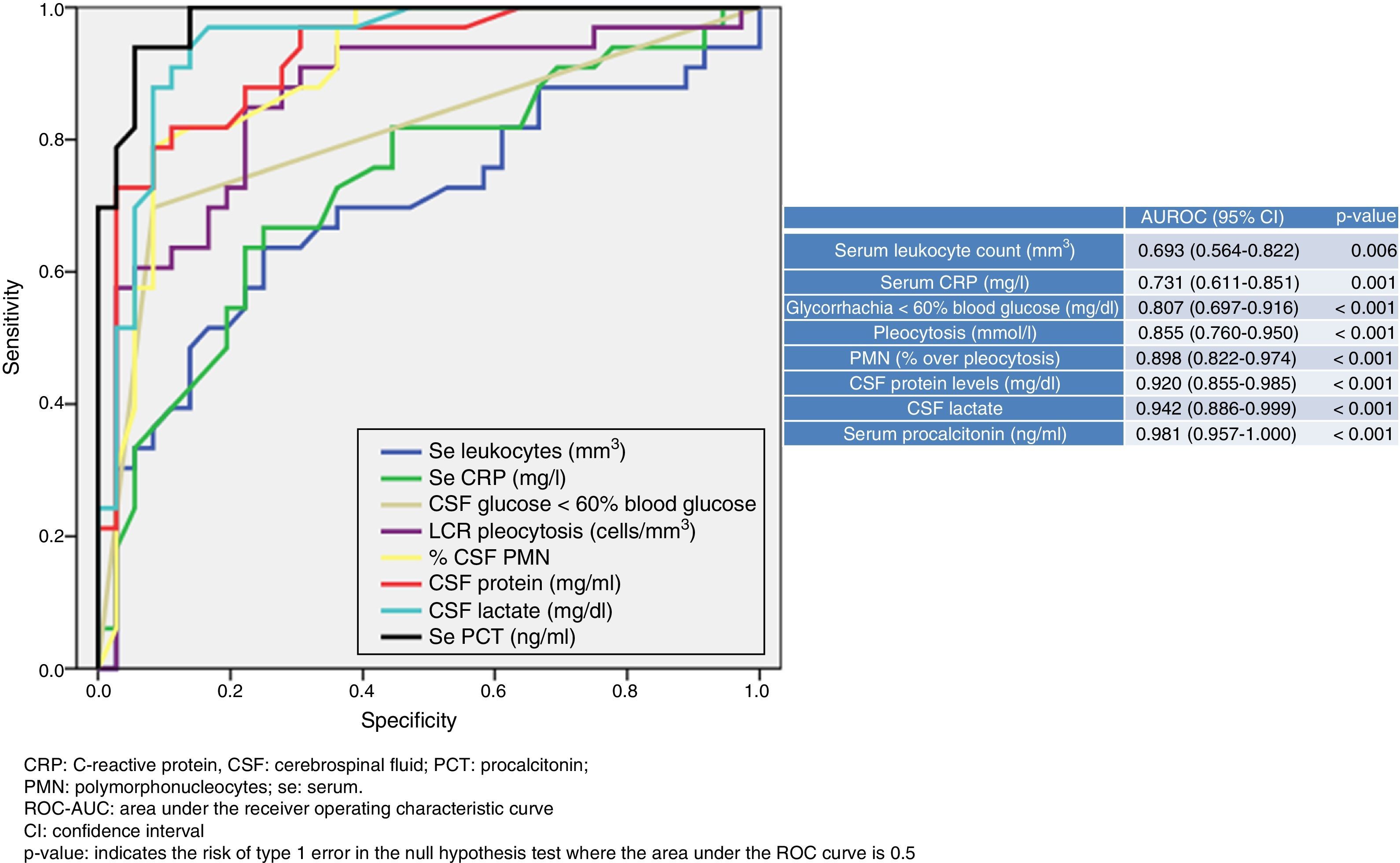
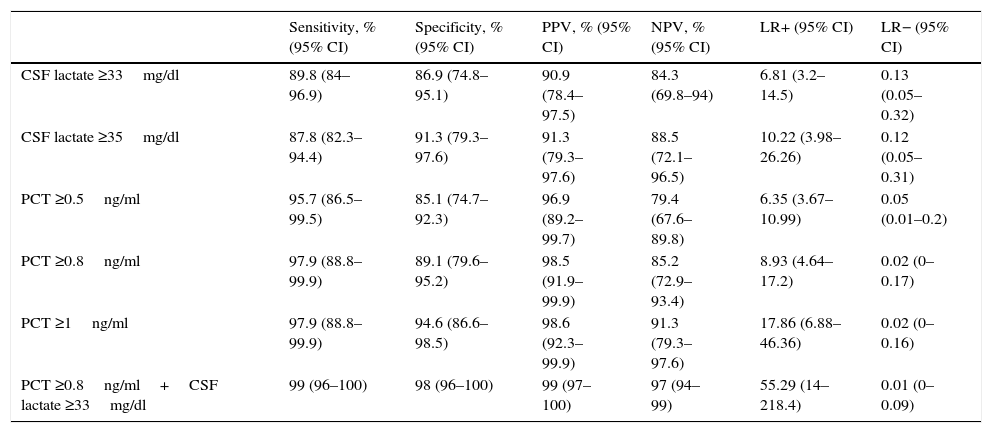
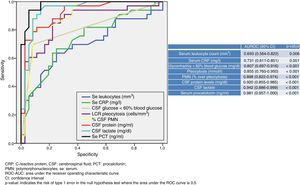
Fig. 1 shows the ROC-AUC values obtained for the emergency department laboratory tests to predict the existence of BM in the overall study population. Of the variables analysed in serum, PCT was found to yield the highest diagnostic performance and the best ROC-AUC of 0.981 (95% CI: 0.957–1; p<0.001). The PCT cut-off point with greatest diagnostic capacity was 0.8ng/ml. Table 4 describes the SEN, SPE, PPV, NPV, LR+ and LR− values of this cut-off point ≥0.8ng/ml, as well as of the cut-off points ≥0.5ng/ml and ≥1ng/ml, as they were deemed to be of significant clinical interest.14,16 In terms of the CSF analysis, lactate concentration was the individual variable with the best ROC-AUC, with 0.942 (95% CI: 0.886–0.999; p<0.001). The most effective cut-off point found was a concentration ≥33mg/dl (all the diagnostic performance values are shown in Table 4, as are the diagnostic performance values for the cut-off point ≥35mg/dl, which is considered to be the reference cut-off point by most authors).
Cut-off points and diagnostic performance for predicting bacterial meningitis in all study patients.
| Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | LR+ (95% CI) | LR− (95% CI) | |
|---|---|---|---|---|---|---|
| CSF lactate ≥33mg/dl | 89.8 (84–96.9) | 86.9 (74.8–95.1) | 90.9 (78.4–97.5) | 84.3 (69.8–94) | 6.81 (3.2–14.5) | 0.13 (0.05–0.32) |
| CSF lactate ≥35mg/dl | 87.8 (82.3–94.4) | 91.3 (79.3–97.6) | 91.3 (79.3–97.6) | 88.5 (72.1–96.5) | 10.22 (3.98–26.26) | 0.12 (0.05–0.31) |
| PCT ≥0.5ng/ml | 95.7 (86.5–99.5) | 85.1 (74.7–92.3) | 96.9 (89.2–99.7) | 79.4 (67.6–89.8) | 6.35 (3.67–10.99) | 0.05 (0.01–0.2) |
| PCT ≥0.8ng/ml | 97.9 (88.8–99.9) | 89.1 (79.6–95.2) | 98.5 (91.9–99.9) | 85.2 (72.9–93.4) | 8.93 (4.64–17.2) | 0.02 (0–0.17) |
| PCT ≥1ng/ml | 97.9 (88.8–99.9) | 94.6 (86.6–98.5) | 98.6 (92.3–99.9) | 91.3 (79.3–97.6) | 17.86 (6.88–46.36) | 0.02 (0–0.16) |
| PCT ≥0.8ng/ml+CSF lactate ≥33mg/dl | 99 (96–100) | 98 (96–100) | 99 (97–100) | 97 (94–99) | 55.29 (14–218.4) | 0.01 (0–0.09) |
CI: confidence interval; CSF: cerebrospinal fluid; LR+: positive likelihood ratio; LR−: negative likelihood ratio; NPV: negative predictive value; PCT: procalcitonin; PPV: positive predictive value.
The cut-off points with the highest diagnostic performance were used (CSF lactate≥33mg/l and PCT≥0.8ng/ml for detecting bacterial meningitis), as well as other cut-off points chosen by the authors (values defined by the laboratory as normal or significant) in order to perform comparisons with other studies.
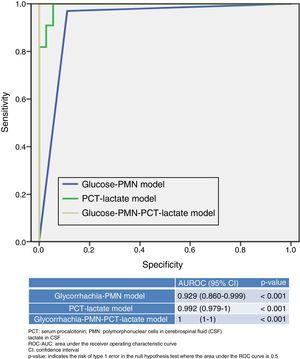
Fig. 2 presents and compares various models that combine different variables used or available in EDs. On the one hand, the new model, generated by combining PCT ≥0.8ng/ml plus CSF lactate ≥33mg/dl, yields a ROC-AUC of 0.992 (95% CI: 0.979–1; p<0.001) and 99% SEN, 98% SPE, a PPV of 99%, an NPV of 97%, an LR+ of 55.29 and an LR− of 0.01, thereby increasing the performance of each individual variable (Fig. 1). Table 4 describes the SEN, SPE, PPV, NPV, LR+ and LR− of this new model.
On the other hand, the model generally used by clinicians, which combines the proportion of PMN>50% in CSF with glycorrhachia (mg/dl) <60% of simultaneous blood glucose, offers poorer performance (ROC-AUC of 0.929 [95% CI: 0.860–0.999]; p<0.001) than the new model referred to above (PCT plus lactate). Combining both models (PCT≥0.8ng/ml plus CSF lactate≥33mg/dl together with CSF PMN>50% and glycorrhachia [mg/dl] <60% of simultaneous blood glucose) yields a ROC-AUC of 1, with a SEN of 100% and a SPE of 100% to predict BM, thereby increasing the yield of each individual variable and of both models.
DiscussionThe results of our study confirm that, after the emergency assessment performed on patients with AM, the significantly correlated independent factors with the greatest predictive capacity of bacterial aetiology are lactate, proportion of CSF PMN and glycorrhachia, as well as serum PCT concentrations.21,22 These 4 factors, which can be routinely tested in EDs,23 comprise an obvious diagnostic approach, the compulsory implementation of which would enable BM to be suspected and patients to be immediately referred to the most appropriate resources or department.
The usual clinical manifestations and symptoms do not offer sufficient sensitivity or specificity to identify a possible case of BM.8,9 What is more, they tend to be more nonspecific in neonates,10,24 the elderly,25 immunosuppressed patients, diabetics and other patients who are particularly susceptible to severe infection.26 This is supported by our study, in which fever, headache, signs of meningeal irritation and stiff neck were not suggestive of bacterial meningitis in the ED. Only altered level of consciousness (which also commonly manifests in cases of viral meningoencephalitis) was higher in BM cases than in VM (67.9% vs 22.9%, p<0.001). We also found significant differences upon comparison of the sepsis, severe sepsis and septic shock criteria (92.5% vs 65.7%, 32% vs 0% and 15% vs 0%, respectively). These data are consistent with recent epidemiological studies of infection,2 and specifically central nervous system infection,3 in EDs.
There is therefore an argument to be made to equip EDs with the appropriate and instant resources to be able to suspect and distinguish between BM and VM cases. This would help to guide the essential decision-making process that is so vital for patient prognosis and outcome from initial contact with the hospital or triage,6 and to distinguish between sepsis and meningitis: extraction of cultures and samples for microbiology, early and appropriate administration of antibiotics, referral to the appropriate department, etc. These resources include inflammatory and infection biomarkers,13,14,27 and particularly PCT in blood tests due to its greater diagnostic performance.15,16,24,25,28–30 In terms of CSF analysis, recent publications have found lactate to have the highest predictive diagnostic value of BM, ahead of cell count (pleocytosis), the proportion of PMN or existing glycorrhachia.17,18
In line with other authors,15,16,24,25,28–30 our study confirms PCT's very high predictive capacity for BM when BM is suspected in the diagnosis of possible cases of AM in the ED. Its diagnostic power is significantly higher than leucocyte count or CRP concentration. Furthermore, the diagnostic capacity of CRP is known to diminish as the age of the patient increases (it is far less effective in the elderly), whereas PCT's performance remains consistently high regardless of age.30 This should be taken into account when assessing and interpreting biomarker concentrations, particularly because patients with BM tend to be older than patients with VM (56 years [SD 22] versus 37 years [SD 20]), as also attested to in our study. The establishment of optimal cut-off points continues to be a subject of much debate and varies significantly from study to study (particularly dependent on sample size and PCT technique used), ranging from 0.2 to 5ng/ml (all of which obtained >90% SEN and SPE).15,16,24,25,28–30 One of the most significant studies, which was conducted by Viallon et al.29 on 254 patients with AM (35 BM and 181 VM), concluded that the greatest diagnostic power is obtained with a PCT cut-off point ≥0.28ng/ml, yielding 95% SEN, 100% SPE, a PPV of 100% and a NPV of 97%, with a ROC-AUC of 0.99 (95% CI: 0.99–1). These results are very similar to those found in our study, except that our cut-off point to achieve similar SEN, SPE, PPV and NPV values was higher (PCT≥0.8ng/ml). A recently-published meta-analysis16 confirmed the excellent results of our study, obtaining 90% SEN, 98% SPE and an OR of 287 (95% CI: 55–1409) for PCT. These results suggest that, for the safety of the patient, a possible case of BM should be suspected in the ED (if not already suspected) when initial PCT is >0.25–0.5ng/ml, thereby enabling the pertinent microbiological tests to be performed5 and the appropriate antibiotic treatment to be immediately administered.11,31 However, if BM is suspected, it is important to remember that the administration of antimicrobial treatment should not be delayed until complementary investigations or tests have been conducted and their results obtained.8
A novel aspect revealed by our study was the significant differences found when confirmed BM cases were compared to possible partially-treated BM cases, although, as shown above, the differences are minor and not significant in some variables in this case versus those found when comparing BM to VM. When faced with a possible case of partially-treated BM, PCT offers limited support or aetiological guidance as PCT concentrations <0.8ng/ml may be found (especially if the antimicrobial administered is appropriate),14 despite it being a case of BM. It has been reported that serum PCT concentrations may fall at 8–12h in patients receiving antimicrobial treatment.14 This is an important point and must be taken into account to ensure that the necessary and appropriate treatment is administered, as the reliability of blood and CSF tests decreases if antimicrobials have been previously administered.16,32
Although some studies have been conducted to assess and compare the value of CSF PCT versus blood PCT, their power is very small and the results obtained inferior to those obtained for blood PCT in the same studies.33–36 As a result, assessing CSF PCT is not currently recommended.
However, 2 recent meta-analyses17,18 have highlighted the value and reliability of assessing CSF lactate to predict bacterial aetiology in patients with suspected BM. Both studies concluded that CSF lactate offers greater diagnostic accuracy than leucocyte count, glucose or CSF protein to distinguish BM from other aetiologies. As such, a ROC-AUC of 0.945–0.984 was obtained, very similar to that obtained in our study, with 93% SEN, 96% SPE, a LR+ of 22.9 and an OR of 313 (95% CI: 141–698) for a cut-off point≥35mg/dl. These results are practically identical to the results of our study, although with greater range of variation and broader 95% confidence intervals to assess 25 studies17 and 33 studies,18 respectively. Both studies found 35mg/dl to be the best-performing cut-off point, compared to 33mg/dl in our study. As a result, BM should be considered in the ED when CSF lactate is >33–35mg/dl.
Similarly, serum lactate, which is the best marker of tissue hypoperfusion/hypoxia, is included in the prognostic assessment and clinical severity guidelines of patients with sepsis, severe sepsis/septic shock in EDs,20 but is not related to bacterial aetiology.14
As a novel aspect of our study, we have compared the bacterial infection diagnostic capacity of the biomarkers and the emergency department lab tests that are analysed in the CSF. This is because we believe it is useful in clinical practice to know both the individual capacity of each test, as well as the excellent performance that results from combining serum PCT with CSF lactate, as suggested by Viallon et al.29 in their study, to improve yet further the predictive capacity of both variables. As such, whether we only obtain serum samples and not CSF due to the failure to conduct a lumbar puncture (as occurs in 10–30% of cases due to contraindication or non-availability of the technique),5 or if both CSF and blood samples are obtained, the accuracy and predictive capacity of BM with the resources we have available is excellent. PCT yields a ROC-AUC of 0.981, with an OR of 46 and for a cut-off point ≥0.8ng/ml with 97% SEN and a PPV of 89%. If not already performed or considered, this finding “would indicate the performance of a lumbar puncture”. As mentioned above, should a lumbar puncture not be possible, BM would have to be heavily suspected in the ED. In addition, the CSF lactate, proportion of PMN and glycorrhachia results, once known, are the tests that can individually identify BM.
We believe that the most influential finding of our study is the increased predictive value obtained by simply combining the most effective blood and CSF tests. This will improve diagnostic accuracy and facilitate prompt and appropriate antimicrobial administration, microbiological cultures and referral to the appropriate department.8,14 As such, the better and higher diagnostic predictive capacity offered by this new combined model (serum CRP plus CSF lactate) compared to the predictive capacity offered by the classic PMN proportion and glycorrhachia model, as well as the potential to achieve 100% SEN and SPE if the 4 variables are taken into account, is extremely relevant.
In terms of appropriate hospital admission and depending on the resources and baseline status of each patient, AM patients with PCT<0.5ng/ml and CSF lactate<33mg/dl could be admitted to a short-stay unit or to the ward until the microbiological diagnosis is confirmed, provided that the patient's clinical status is compatible (i.e. patients without severe sepsis criteria), and without ruling out the possibility of partially-treated BM, as referred to above. Patients with PCT≥0.5ng/ml and/or lactate ≥33mg/dl should be admitted to units that provide intensive and continuous clinical and analytical monitoring.37,38
Our study had several limitations, principally the fact that it was a single-centre study coupled with its limited sample size (beta error cannot be ruled out). This meant that limited data were available for some comparisons, which certainly could have led to the cut-off points and performances obtained being maximised or minimised. Another limitation of our study was the lack of a disease-free control group, which would have conferred a higher degree of internal validity. The selection of variables could also have been more comprehensive, as the CSF opening pressure was not included owing to the lack of recorded data. As such, the variables analysed in the CSF, the cut-off points, the values adopted as normal and the dichotomised variables were all arbitrarily chosen, as detailed in the methodology. This must be taken into account when drawing conclusions from the results and comparing them with other studies. An analysis of the complications (encephalitis, seizures, etc.), which may have provided more pertinent information to the study, was also not taken into account.
Despite these limitations, we believe that our study reflects clinical practice in our EDs in terms of suspecting and confirming AM. It also highlights the role that serum PCT and CSF lactate, glycorrhachia and proportion of PMN measurements could play as a predictive indicator of BM. However, future external validation studies with greater power and larger samples are needed.
In conclusion, the inclusion of serum PCT in the requested lab tests, together with CSF lactate, glycorrhachia and PMN measurements in the initial assessment of patients suspected of having AM in the ED, have been shown to be useful predictive indicators of BM versus VM.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank Dr Eva Heredero Gálvez and the Microbiology Department of the Complejo Hospitalario de Toledo for their assistance in providing the information pertaining to the complementary tests and microbiological cultures. We would also like to thank the Clinical Chemistry and Clinical Analysis Department for their collaboration in the collection and analysis of biomarker and cerebrospinal fluid samples.
Please cite this article as: Morales-Casado MI, Julián-Jiménez A, Lobato-Casado P, Cámara-Marín B, Pérez-Matos JA, Martínez-Maroto T. Factores predictores de meningitis bacteriana en los pacientes atendidos en urgencias. Enferm Infecc Microbiol Clin. 2017;35:220–228.