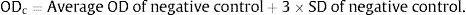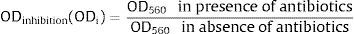Device associated infections caused by Staphylococcus aureus in hospitalised patients is a serious healthcare problem. The present study was designed to determine the prevalence of biofilm-producing MRSA in device-associated infections.
MethodsDevice-associated S. aureus strains (n=200) obtained from two tertiary care hospitals in Mysuru city, India were screened for biofilm production, antibiotic resistance, Panton-Valentine Leucocidin genes, SCCmec-types, spa-types, and intercellular adhesion (icaAD) dependent and independent genes. The efficacy of antibiotics (linezolid, vancomycin and rifampicin) on biofilms was studied using MTT assay, and the results were correlated with the occurrence of ica-dependent and independent factors.
ResultsMultidrug resistance was observed in 155 strains (77.5%), and 124 strains (62%) were identified as biofilm producers. Methicillin resistance was identified in 145 strains (72.5%), and SCCmec typing of these isolates revealed high prevalence of type IV and type V. They also showed increased prevalence of pvl gene. icaAD was identified in 65 isolates, with 37 isolates showing both icaAD and ica-independent genes. spa types t852 and t657 were predominantly observed in MRSA isolates. Those isolates that had both ica-dependent and ica-independent genes showed more resistance to the screened antibiotics than the ica-dependent alone.
ConclusionThis study reports a high prevalence of SCCmec type IV and V in biofilm producing S. aureus strains isolated from device-associated infections. Increased prevalence of pvl in SCCmec types IV and V strains suggests the role of community associated S. aureus in device-associated infections. The simultaneous presence of ica-dependent and independent genes increased the antibiotic resistance in established biofilms. Thus, S. aureus on medical devices is a potential risk for patients.
Las infecciones asociadas a dispositivos médicos causadas por Staphylococcus aureus en pacientes hospitalizados son un problema importante. En el presente trabajo se estudia, en cepas de infecciones asociadas a dispositivos médicos, la prevalencia SARM productores de biopelículas y sus tipos SCCmec.
MétodosSe usaron 200 cepas de S. aureus de infecciones de dispositivos médicos obtenidas de 2 hospitales terciarios de Mysuru, India. Se estudió la producción de biopelículas, los genes de la leucocidina de Panton-Valentine, los tipos SCCmec, los tipos de spa y los genes de adhesión intracelular (icaAD) dependientes e independientes. Se estudió la eficacia de linezolid, vancomicina y rifampicina en las biopelículas por un ensayo MTT y los resultados se correlacionaron con la presencia de genes ica dependientes e independientes.
ResultadosCiento veinticuatro cepas (62%) producían biopelículas y se observó multirresistencia antibiótica en 155 (77,5%). Eran resistentes a meticilina 145 cepas (72,5%) y en su tipificación SCCmec se observó alta prevalencia de los tipos iv y v. Estas cepas tenían una prevalencia superior de gen pvl a las no resistentes a meticilina. icaAD se identificó en 65 aislados, de los que 37 mostraron simultáneamente genes ica dependientes e independientes. Los spa tipos t852 y t657 se observaron predominantemente en las cepas de SARM. Los aislados que tenían a la vez genes ica dependientes e ica independientes presentaban mayor resistencia a los antibióticos probados que los que tenían solo ica dependientes.
ConclusiónEl presente estudio informa de una alta prevalencia de SARM de los SCCmec tipos iv y v en cepas de S. aureus productoras de biopelículas. La elevada prevalencia del gen pvl en las cepas de los SCCmec IV y V sugiere el papel de los S. aureus comunitarios en las infecciones asociadas a estos dispositivos. La presencia simultánea de genes ica dependientes e independientes aumenta la resistencia a antibióticos en las biopelículas establecidas. Por todo ello, las cepas de S. aureus en dispositivos médicos son un riesgo potencial para los pacientes.
Medical devices have been extensively used in health care; but they are known to promote bacterial adherence through biofilms. The contact of medical devices with the extracellular matrix proteins in patients, can trigger biofilm production and cause device-associated infections. Staphylococcus aureus, an important pathogen of community and hospital acquired infections, has been frequently reported to form biofilm on indwelling devices such as orthopaedic implants, urinary catheters, central venous catheters, peripheral venous catheters, endotracheal tubes, cardiac prosthetic valves, contact lens and on surgical sites.1
Biofilms formed by S. aureus on orthopaedic implants are reportedly known to cause increased morbidity in patients when compared to the infection on other implants.2 But S. aureus biofilm on peripheral venous catheters (PVC) and central venous catheters (CVC) is also a major problem as it leads to blood stream infections.3 Tracheobronchial secretions in the intubated endotracheal tube acts as a sovereign risk factor for ventilator-associated pneumonia (VAP). These secretions promote S. aureus to form biofilm on the inner surface of endotracheal tube. Recent study on endotracheal tube mediated VAP has shown the prevalence of 22% of biofilm positive S. aureus.4S. aureus is also accounted for infective endocarditis in patients with cardiac prosthesis.5 A multi-national study comprising 1779 patients with infective endocarditis has shown high prevalence of 31.4% of S. aureus in these infections.6 Thus, the plethora of biofilm associated infections caused by S. aureus is unimaginable and surveillance studies to monitor the spread of S. aureus in hospitals are warranted.
Production of biofilm by S. aureus is primarily mediated by the intercellular adhesin operon (ica operon) which codes for the synthesis of polysaccharide intercellular adhesin (PIA).7 Biofilm can also be triggered by intercellular adhesion independent factors such as biofilm associated protein (Bap), clumping factor A and B (ClfA and ClfB), Fibronectin binding proteins A and B (FnBpA and FnBpB), Staphylococcus aureus surface protein A (Spa), cidAB, Staphylococcus aureus surface proteins G and C.8–10 So based on the presence or absence of intercellular adhesion genes, biofilm promoting genes in S. aureus are broadly classified into ica-dependent and ica-independent factors.
Apart from biofilm production, upsurge in S. aureus infections is mainly due to the acquisition of resistance to antibiotics belonging to beta-lactam group.11 One such antibiotic is methicillin and the resistance to this antibiotic was reported within one year of its introduction. Methicillin Resistant Staphylococcus aureus (MRSA) has now emerged as a global pathogen in hospital- and community-associated infections. Like many other countries, even in India the prevalence of MRSA has been increased.12 Resistance to methicillin in S. aureus is mainly mediated by the mecA gene carried on a mobile genetic element staphylococcal cassette chromosome mec (SCCmec).13 These are extremely diverse elements and based on the structural organization, they have been broadly classified into SCCmec type I to SCCmec XI.14 Recent studies have shown that, in clinical isolates, the presence or absence of mecA gene influences the expression of biofilm phenotype.15 Methicillin susceptible strains are known to show enhanced biofilm production by expressing PIA16; whereas, methicillin resistant strains formed biofilm in an ica-independent manner by secreting surface proteins or by releasing extracellular DNA.17 These data indicates that there is an increasing need to study the occurrence of methicillin resistance in biofilm producing S. aureus.
MRSA are broadly classified into community associated-MRSA (CA-MRSA) and hospital associated-MRSA (HA-MRSA). A typical CA-MRSA strain will show heightened susceptibility to antimicrobial agents, except for antibiotics belonging to beta-lactam group. This suggests that most of the CA-MRSA strains carry SCCmec type IV and V as they consists of smaller genetic island when compared to SCCmec I–III which are mainly found in HA-MRSA strains.18 Recent study from our lab has also shown the emergence of CA-MRSA of sequence type (ST) 2371 as a major clone among the clinical isolates collected from a tertiary care hospital in Mysuru, India.19 These data clearly demonstrate the need for SCCmec-typing in device-associated S. aureus.
Presence of Panton-Valentine Leukocidin (pvl) gene is considered as a marker for CA-MRSA strains.20 It is interesting to note that, pvl is less frequently reported in biofilm associated infections.21 Hence, screening of pvl gene in device-associated isolates is necessary.
The present study was carried with an objective to identify the distribution of SCCmec types, spa types, pvl, antibiotic resistance, biofilm production and co-occurrence of ica-dependent and independent factors in device associated isolates. To the best of our knowledge, this is the first prevalence report on device-associated infections from Mysuru city, India which has a population of 3,001,127.22
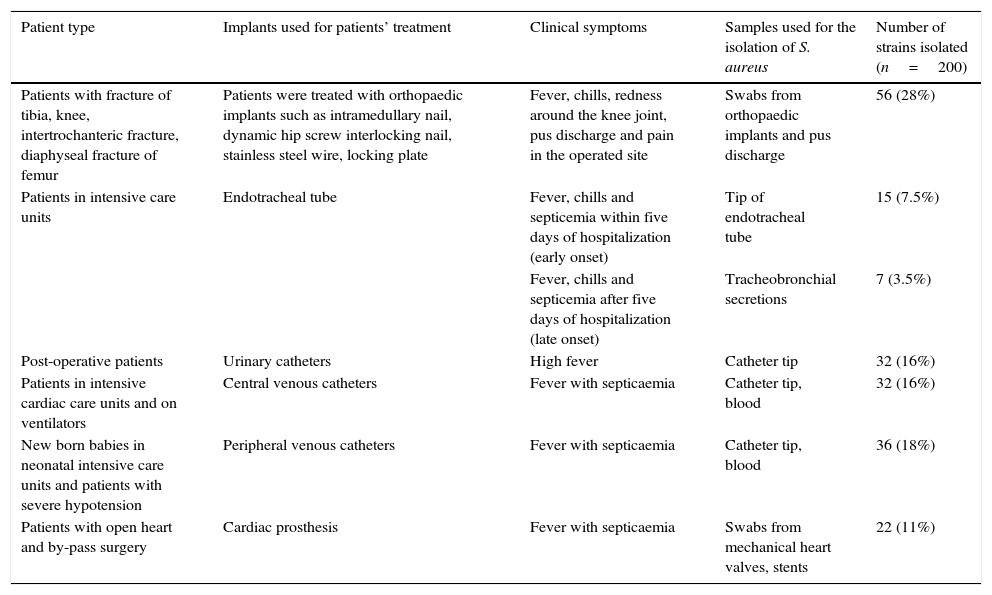
Materials and methodsSample collectionThis is a cross-sectional study, wherein 200 (n=200) clinical S. aureus strains isolated from infected implants over a period of one year (January 2014 to January 2015), were studied. These strains were obtained from the Department of Microbiology at Jagadguru Sri Shivarathreeswara (JSS) Medical Hospital and Krishnarajendra (KR) Hospital located in Mysuru, India. JSS medical hospital is a tertiary care centre with 1800 beds and it has 37 super-speciality units. The KR hospital, another tertiary care hospital in Mysuru, has 1330 bed capacity and has more than 20 super-speciality units. Initial confirmation of S. aureus was done by the hospital staff using coagulase test, mannitol fermentation test and DNAse test. Later, molecular confirmation of S. aureus up to species level was done by PCR using the reported primers.23 The American Association of Orthopaedic surgeons has classified orthopaedic prosthetic infections into four types: Type I – any two intraoperative cultures showing positive results, Type II – infection occurring within one month after surgery, Type III – infection in a working prosthesis caused by haematogenous seeding, Type IV – infection persisting in a patient for more than one month.24 So based on the types, strains were isolated from orthopaedic implants. Types of patients, clinical conditions and the samples used for the isolation of S. aureus are shown in Table 1.
Types of patients, clinical conditions and samples used for the isolation of S. aureus.
| Patient type | Implants used for patients’ treatment | Clinical symptoms | Samples used for the isolation of S. aureus | Number of strains isolated (n=200) |
|---|---|---|---|---|
| Patients with fracture of tibia, knee, intertrochanteric fracture, diaphyseal fracture of femur | Patients were treated with orthopaedic implants such as intramedullary nail, dynamic hip screw interlocking nail, stainless steel wire, locking plate | Fever, chills, redness around the knee joint, pus discharge and pain in the operated site | Swabs from orthopaedic implants and pus discharge | 56 (28%) |
| Patients in intensive care units | Endotracheal tube | Fever, chills and septicemia within five days of hospitalization (early onset) | Tip of endotracheal tube | 15 (7.5%) |
| Fever, chills and septicemia after five days of hospitalization (late onset) | Tracheobronchial secretions | 7 (3.5%) | ||
| Post-operative patients | Urinary catheters | High fever | Catheter tip | 32 (16%) |
| Patients in intensive cardiac care units and on ventilators | Central venous catheters | Fever with septicaemia | Catheter tip, blood | 32 (16%) |
| New born babies in neonatal intensive care units and patients with severe hypotension | Peripheral venous catheters | Fever with septicaemia | Catheter tip, blood | 36 (18%) |
| Patients with open heart and by-pass surgery | Cardiac prosthesis | Fever with septicaemia | Swabs from mechanical heart valves, stents | 22 (11%) |
Biofilm screening by crystal violet method was performed using a modified protocol as described earlier.25 Single colony of the isolate was inoculated to 25ml trypticase soy broth with 0.5% glucose (TSB+0.5% G) and incubated at 37°C for 8–10h until 0.5 OD is obtained. This culture was re-inoculated to fresh TSB+0.5% G and incubated for 3–4h to get 0.03 OD. 200μl of this culture was inoculated to 96 wells flat bottom microtitre plate in triplicate and incubated at 37°C for 48h. Staphylococcus aureus ATCC 35556 was used as positive control and 3 wells with TSB+0.5% G alone were kept as negative control. After incubation, plates were washed thrice with 0.15M sterile PBS and stained with 0.1% crystal violet (HiMedia Labs, Mumbai, India) for 30min at room temperature. Stained microtitre plates are washed thrice with sterile distilled water and dried in hot air oven at 45°C. The bound dye was re-solubilised by adding 160μl of 33% (v/v) glacial acetic acid to each well and kept for 30minutes at room temperature. The re-solubilised dye was transferred to a new microtitre plate and OD was measured at 570nm in a spectrophotometer (Thermo-Fischer Scientific). Based on the obtained OD values, quantification of biofilm strength was performed using the following formula.
At first the cut off OD (ODc) was calculated using the formula
The ODc was compared with the OD of samples (ODs) and the strength of biofilm production observed in each isolate was assessed using the following formulaAntibiogram and MIC of biofilm positive isolatesKirby-Bauer agar disc diffusion method was performed to determine the antibiogram of device associated strains.26 Antibiotics used were cefoxitin, erythromycin, gentamicin, rifampicin, trimethoprim-sulphomethoxazole, tetracycline, clindamycin, linezolid and ciprofloxacin. The susceptibility to antibiotics was assessed as per the Clinical Laboratory Standard Institute (CLSI) guidelines.27 MIC for oxacillin was calculated by agar-dilution method and the results were interpreted as per the CLSI guidelines.27
SCCmec-typingMRSA isolates were screened for different mec types by multiplex PCR using the conditions as previously described.28 Accuprime Taq DNA polymerase (Invitrogen, USA) was used for the multiplex-PCR. The control strains used for typing were S. aureus COL (type I), S. aureus BK2464 (type II), S. aureus ANS46 (type III), S. aureus MW2 (type IV), S. aureus WIS (type V) and S. aureus HDE288 (type VI).
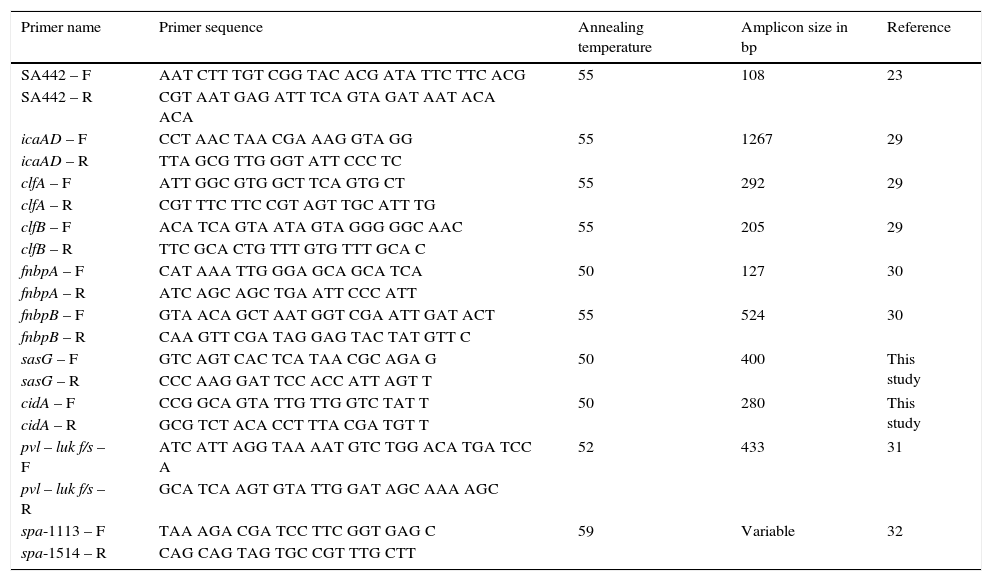
Molecular screening of pvl, ica-dependent and independent factorsMolecular screening of ica-dependent, ica-independent and pvl-luk f/s genes were done by PCR. DNA was isolated using QIAamp DNA mini kit (Qiagen Cat. No. 51306) and the isolated DNA was used for PCR. The reaction was carried out in a 25μl microfuge tube containing 2μl of DNA, 11μl of 2X PCR master mix (Cat. No. Beta Midsci™) and 12μl of distilled water. Primers used for the detection of S. aureus, pvl, ica-dependent and independent factors are listed in Table 2 with the respective annealing temperature and amplicon size. PCR programme was set with an initial denaturation at 94°C for 5min followed by 35 cycles of 30s of denaturation at 94°C, 30s of annealing and 60s of extension at 72°C. Final extension at 72°C for 10min was set as the last step of PCR programme. PCR was carried out in Eppendorf Master Cycler. 10μl of amplified PCR products along with 1kb DNA marker were loaded on 1.5% agarose gel and electrophoresis was performed. Amplicons were analyzed by using a gel documentation system.
Primer sequence, annealing temperature and amplicon size used for molecular characterization.
| Primer name | Primer sequence | Annealing temperature | Amplicon size in bp | Reference |
|---|---|---|---|---|
| SA442 – F | AAT CTT TGT CGG TAC ACG ATA TTC TTC ACG | 55 | 108 | 23 |
| SA442 – R | CGT AAT GAG ATT TCA GTA GAT AAT ACA ACA | |||
| icaAD – F | CCT AAC TAA CGA AAG GTA GG | 55 | 1267 | 29 |
| icaAD – R | TTA GCG TTG GGT ATT CCC TC | |||
| clfA – F | ATT GGC GTG GCT TCA GTG CT | 55 | 292 | 29 |
| clfA – R | CGT TTC TTC CGT AGT TGC ATT TG | |||
| clfB – F | ACA TCA GTA ATA GTA GGG GGC AAC | 55 | 205 | 29 |
| clfB – R | TTC GCA CTG TTT GTG TTT GCA C | |||
| fnbpA – F | CAT AAA TTG GGA GCA GCA TCA | 50 | 127 | 30 |
| fnbpA – R | ATC AGC AGC TGA ATT CCC ATT | |||
| fnbpB – F | GTA ACA GCT AAT GGT CGA ATT GAT ACT | 55 | 524 | 30 |
| fnbpB – R | CAA GTT CGA TAG GAG TAC TAT GTT C | |||
| sasG – F | GTC AGT CAC TCA TAA CGC AGA G | 50 | 400 | This study |
| sasG – R | CCC AAG GAT TCC ACC ATT AGT T | |||
| cidA – F | CCG GCA GTA TTG TTG GTC TAT T | 50 | 280 | This study |
| cidA – R | GCG TCT ACA CCT TTA CGA TGT T | |||
| pvl – luk f/s – F | ATC ATT AGG TAA AAT GTC TGG ACA TGA TCC A | 52 | 433 | 31 |
| pvl – luk f/s – R | GCA TCA AGT GTA TTG GAT AGC AAA AGC | |||
| spa-1113 – F | TAA AGA CGA TCC TTC GGT GAG C | 59 | Variable | 32 |
| spa-1514 – R | CAG CAG TAG TGC CGT TTG CTT |
MRSA isolates were subjected to Spa-typing. The technique involves the sequencing of extremely variable X region in the staphylococcal protein A. Amplification of variable X region was performed using the primers described in Table 2. Amplified products were sequenced and the spa types were identified using Bionumerics version 6 software (Applied maths, Belgium).
MTT assay for the detection of antibiotic resistance in established biofilmsTo check the effect of antibiotics on established biofilms, all the strong biofilm producing strains were subjected to MTT (3-[4,5-dimethyl-2-thiazolyl]-2,5-diphenyl-2H-tetrazolium-bromide) assay. This was performed using the method described previously33 with minor modifications. Based on the CLSI guidelines, resistant break point concentration (RBC) for linezolid, vancomycin and rifampicin was used in the MTT assay. The RBC for linezolid was set as 8μg/ml, Vancomycin 16μg/ml and rifampicin 4μg/ml. Biofilms established on 24 well plates were treated with respective antibiotics for 1, 3 and 5 days. After the antibiotic treatment, wells were emptied and washed thrice with sterile PBS. Then, 100μl of PBS with 1% MTT solution was added and incubated for 2h at 37°C. Later MTT was replaced by 100μl dimethylsulphoxide (DMSO) and incubated for 15min at room temperature. Bacteria with an active electron transport system reduced the tetrazolium salt to a water-soluble purple formazan product. This product was quantified by measuring the OD at 560nm in a microplate reader. The inhibitory effect of antibiotics (ODi) on established biofilms was studied by finding the ratio of OD values in presence of antibiotics and in the absence of antibiotics (control) on 1st, 3rd and the 5th day
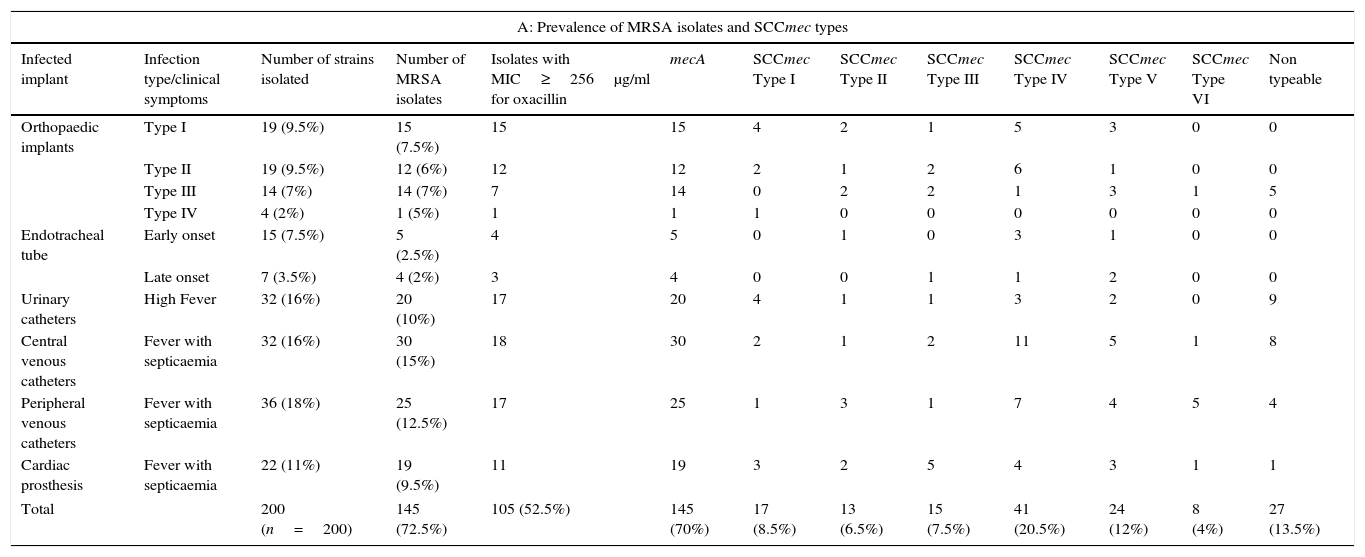
The average OD560 (OD of 1st day, 3rd day and 5th day) of antibiotic treatment was compared with the OD560 values of respective controls of 1st day, 3rd day and 5th day. This represented the average inhibitory effect of a respective antibiotic ODresistance (ODr). Decrease in ODr value indicated greater biofilm inhibition by the respective antibiotic.ResultsPrevalence of MRSA, molecular confirmation of S. aureus and screening of SCCmec typesAll the 200 device-associated isolates obtained from two hospitals were confirmed as S. aureus by PCR. High prevalence of SCCmec types IV and V was observed. Some of the strains were found to be non-typeable. The prevalence of MRSA and distribution of SCCmec types among device associated strains are shown in Table 3A.
Clinical and molecular characteristics of S. aureus strains isolated from infected medical implants.
| A: Prevalence of MRSA isolates and SCCmec types | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Infected implant | Infection type/clinical symptoms | Number of strains isolated | Number of MRSA isolates | Isolates with MIC≥256μg/ml for oxacillin | mecA | SCCmec Type I | SCCmec Type II | SCCmec Type III | SCCmec Type IV | SCCmec Type V | SCCmec Type VI | Non typeable |
| Orthopaedic implants | Type I | 19 (9.5%) | 15 (7.5%) | 15 | 15 | 4 | 2 | 1 | 5 | 3 | 0 | 0 |
| Type II | 19 (9.5%) | 12 (6%) | 12 | 12 | 2 | 1 | 2 | 6 | 1 | 0 | 0 | |
| Type III | 14 (7%) | 14 (7%) | 7 | 14 | 0 | 2 | 2 | 1 | 3 | 1 | 5 | |
| Type IV | 4 (2%) | 1 (5%) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Endotracheal tube | Early onset | 15 (7.5%) | 5 (2.5%) | 4 | 5 | 0 | 1 | 0 | 3 | 1 | 0 | 0 |
| Late onset | 7 (3.5%) | 4 (2%) | 3 | 4 | 0 | 0 | 1 | 1 | 2 | 0 | 0 | |
| Urinary catheters | High Fever | 32 (16%) | 20 (10%) | 17 | 20 | 4 | 1 | 1 | 3 | 2 | 0 | 9 |
| Central venous catheters | Fever with septicaemia | 32 (16%) | 30 (15%) | 18 | 30 | 2 | 1 | 2 | 11 | 5 | 1 | 8 |
| Peripheral venous catheters | Fever with septicaemia | 36 (18%) | 25 (12.5%) | 17 | 25 | 1 | 3 | 1 | 7 | 4 | 5 | 4 |
| Cardiac prosthesis | Fever with septicaemia | 22 (11%) | 19 (9.5%) | 11 | 19 | 3 | 2 | 5 | 4 | 3 | 1 | 1 |
| Total | 200 (n=200) | 145 (72.5%) | 105 (52.5%) | 145 (70%) | 17 (8.5%) | 13 (6.5%) | 15 (7.5%) | 41 (20.5%) | 24 (12%) | 8 (4%) | 27 (13.5%) | |
| B: Prevalence of biofilm regulating genes and PVL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Infected implant | Number of biofilm positive isolates | icaAD | clfA | clfB | fnbpA | fnbpB | sasG | cidA | pvl+SCCmec type IV | pvl+SCCmec type V | pvl+SCCmec type I–III |
| Orthopaedic implants | 56 (28%) | 24 | 11 | 13 | 4 | 10 | 22 | 14 | 15 | 17 | 3 |
| Endotracheal tube | 9 (4.5%) | 4 | 2 | 7 | 5 | 3 | 2 | 0 | 4 | 2 | 0 |
| Urinary catheters | 5 (2.5%) | 2 | 4 | 2 | 4 | 1 | 5 | 0 | 2 | 1 | 0 |
| Central venous catheters | 17 (8.5%) | 7 | 2 | 5 | 10 | 3 | 3 | 2 | 5 | 6 | 1 |
| Peripheral venous catheters | 20 (10%) | 15 | 6 | 3 | 7 | 4 | 10 | 4 | 7 | 1 | 1 |
| Cardiac prosthesis | 19 (9.5%) | 13 | 3 | 7 | 9 | 8 | 5 | 3 | 5 | 7 | 0 |
| Total | 124 (62%) | 65 (32.5%) | 28 (14%) | 37 (18.5%) | 39 (19.5%) | 29 (14.5%) | 47 (23.5%) | 23 (11.5%) | 38 (19%) | 34 (17%) | 5 (2.5%) |
| C: Diversity of Spa-types among device associated MRSA strains | |||
|---|---|---|---|
| SCCmec types | Number of screened isolates | Frequently reported spa types | Number of frequently reported spa types |
| SCCmec type I | 17 | t307 | 15 (88.23%) |
| SCCmec type II | 13 | t018 | 11 (84.61%) |
| SCCmec type III | 15 | t363 | 14 (93.33%) |
| SCCmec type IV | 41 | t852 | 36 (87.8%) |
| SCCmec type V | 24 | t657 | 21 (87.5%) |
| SCCmec type VI | 8 | t172 | 4 (50%) |
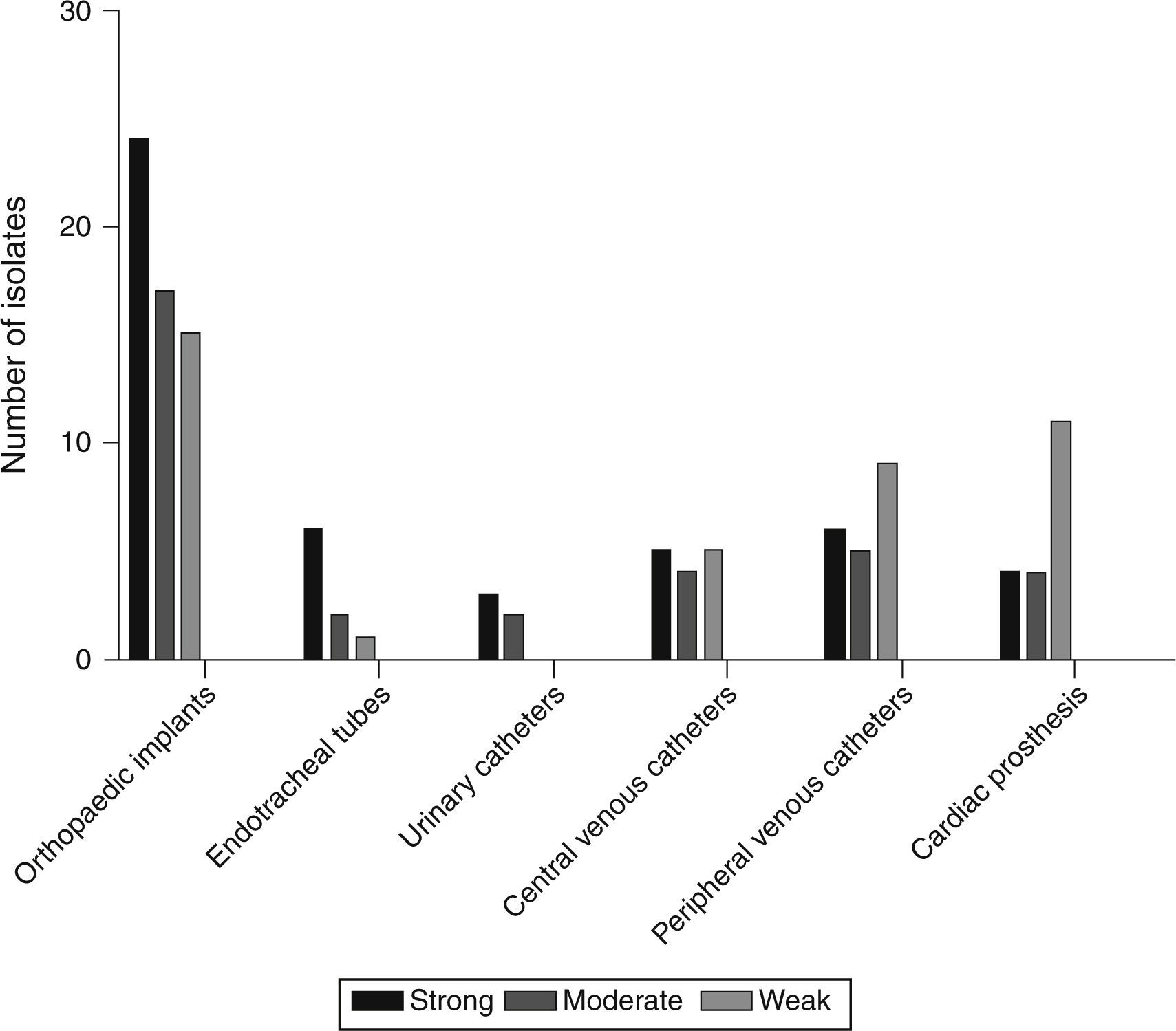
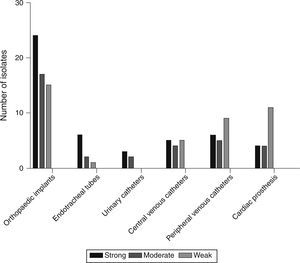
Based on the crystal violet assay, high prevalence of strong biofilm producing strains was observed in orthopaedic implant associated isolates and the lowest was in the isolates from urinary catheters. Strength based characterization and number of strains isolated from infected devices are shown in Fig. 1.
Screening of pvl and biofilm regulating genesBiofilm regulating genes, icaAD, were predominantly found in strong biofilm producing strains. Whereas, ica-independent genes were mainly found in moderate and weak biofilm producers. icaAD alone was found mainly in orthopaedic implants (28%). Interestingly, co-occurrence of ica-dependent and independent factors was higher in orthopaedic implants and peripheral venous catheters. Table 3B describes the occurrence of pvl, ica-dependent and independent genes in biofilm positive strains.
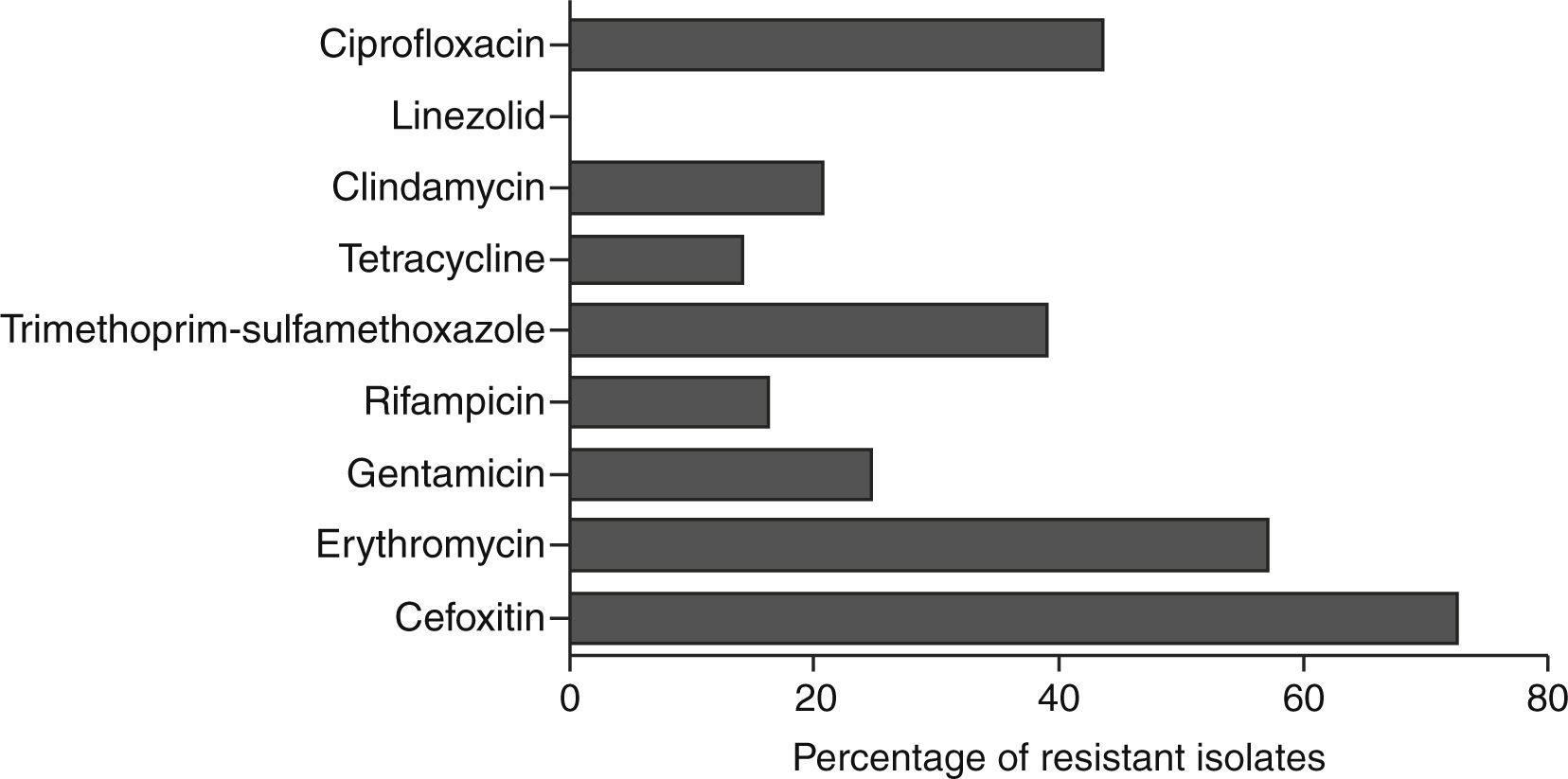
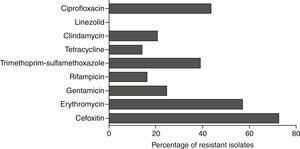
Antibiotic susceptibility testStrains showing resistance to cefoxitin in disc diffusion method were identified as MRSA. These isolates were found to be multi-drug resistant and the percentage of isolates resistant to each antibiotic is shown in Fig. 2.
spa typingOverall 31 different spa types were identified among 200 device associated strains. spa type t852 and t657 were found to be higher among MRSA isolates. Strains with SCCmec type IV mainly belonged to spa type t852 and SCCmec type V belonged to t657. Table 3C describes the distribution of spa types among the device associated strains.
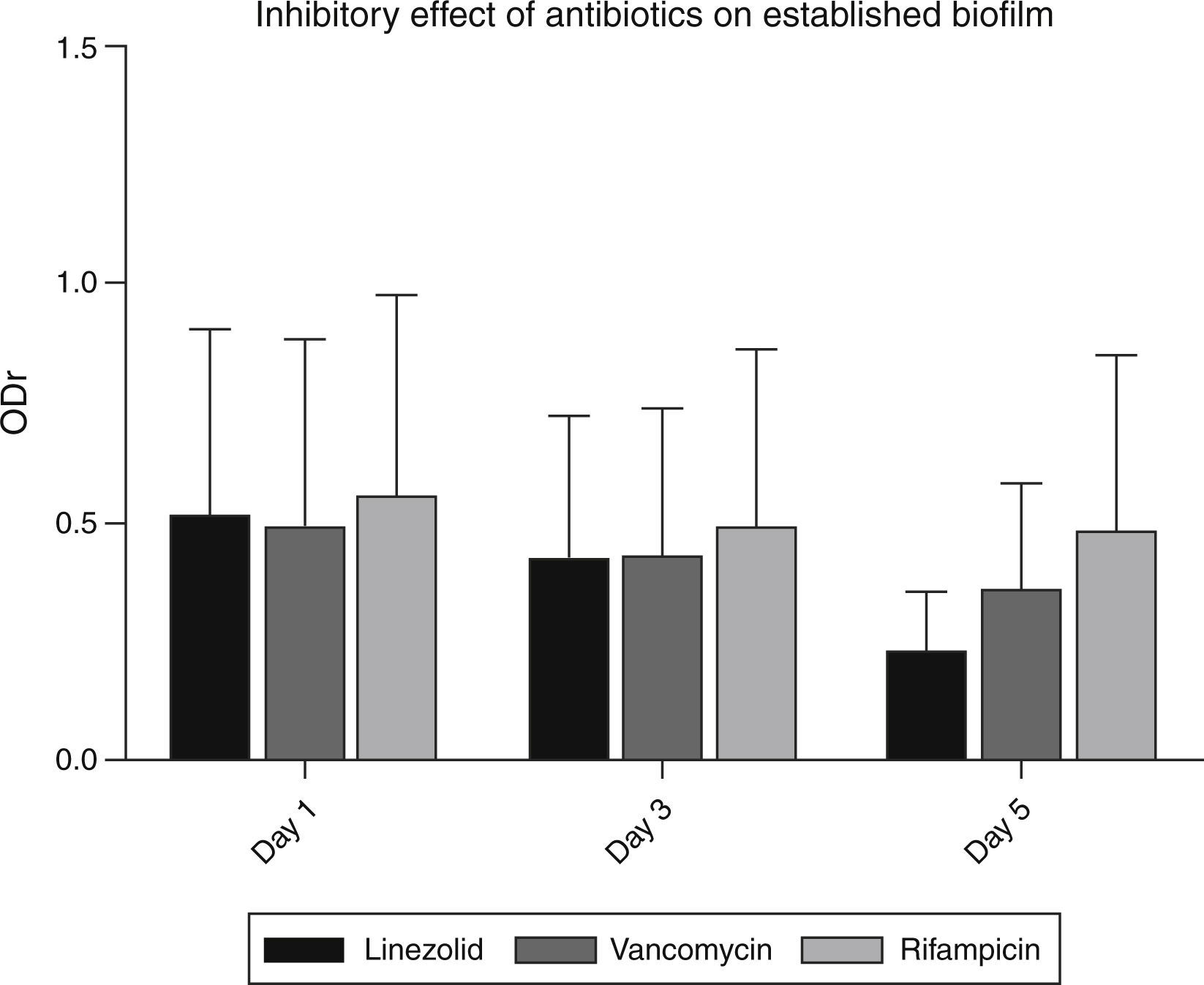
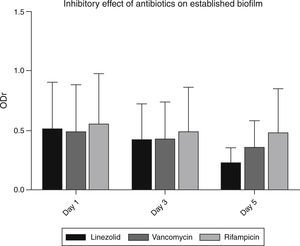
Detection of antibiotic resistance in established biofilmsVancomycin and linezolid at a concentration of 16μg/ml and 8μg/ml respectively, were effective in inhibiting established strong biofilms (Fig. 3). Among 48 strong biofilm producers, these two antibiotics controlled biofilm production in 33 strains. But in remaining 9 strains, the inhibitory effect of these antibiotics was less. These strains, possessed both icaAD (ica-dependent) and all the ica-independent factors (fnbpA, fnBpB, cidA, clfA, clfB and sasG).
DiscussionThis is a first report on the prevalence and molecular profiling of device-associated S. aureus infections from Mysuru, India. Prevalence of device-associated infections was exceptionally high in isolates obtained from orthopaedic implants and peripheral venous catheters. In the present study, isolates from orthopaedic implants were able to form strong biofilms. This data suggests the increased risk of infections on orthopaedic implants through biofilm formation. Among the four types of orthopaedic implant associated infections, highest prevalence was observed in type I (9.5%) and type II (9.5%). The increased prevalence of orthopaedic device-associated infections, especially in type I and type II, warrants the need for proper hygiene practice during surgery.
Prevalence of peripheral venous catheter infections caused by S. aureus was moderate and the majority of strains showed weak biofilm phenotype. This could be due to their low duration of implantation. But some strains demonstrated strong biofilm phenotype, which foresee the essential role of S. aureus in PVC-associated infections. Device-associated infections were not limited to orthopaedic and peripheral venous catheters but were also observed in urinary catheters, central venous catheters and cardiac prosthesis. Their prevalence was comparatively low and they exhibited strong, moderate and weak biofilm phenotype.
Strains were further characterized for antibiotic susceptibility, SCCmec types, pvl, spa-types and biofilm regulating genes. The majority of isolated strains were resistant to methicillin (72.5%) and erythromycin (57%). Interestingly, resistance to gentamicin, clindamycin, rifampicin and tetracycline was mainly observed in the strains isolated from orthopaedic implants, cardiac prosthesis and peripheral venous catheters.
Minimal Inhibitory Concentration ≥256μg/ml for oxacillin was observed in 105 (52.5%) strains. This high occurrence of extremely resistant MRSA strains in device-associated infections is alarming and thus screening for SCCmec types was performed. The recent trend in the epidemiology of CA-MRSA has shown the increased infiltration of these strains to hospitals.34 In the present study, high prevalence of SCCmec type IV (20.5%) and V (12%) among device-associated strains was observed. This could be due to the possible persistence of CA-MRSA strains in these hospitals. Occurrence of pvl in clinical strains is a useful marker to identify CA-MRSA strains. Thus, pvl screening for all the MRSA isolates was performed and it was found in 38 SCCmec type IV and 34 SCCmec type V strains. This increased occurrence of pvl and SCCmec type IV and V, suggests the possible intrusion of CA-MRSA strains in hospitals.
The diversity of S. aureus strains in device-associated infections, was studied by spa-typing for MRSA isolates. spa types t852 and t657 were frequently observed in SCCmec types IV and V. Strains with SCCmec types I, II, III and VI mainly displayed t307, t018 and t363, t172 Spa types respectively. spa types t018, t852 and t657 are reported to belong to the clonal complex CC30, CC22 and CC1 respectively.35 A recent study from Bangalore, south India, also showed the occurrence of t852, t3387 and t657 spa types in CA-MRSA strains.36 This clearly indicates the distribution of these spa types in this geographical region.
Numerous predisposing factors have been identified in the formation of biofilm on device-associated infections. One such factor is the implant itself. But biofilm on an implant can also be interceded by the duration of implantation, patient's health condition, antibiotic therapy and the presence or absence of biofilm regulating genes. In this context, biofilm positive strains (MRSA and MSSA) were characterized for the presence or absence of intercellular adhesion-dependent and independent genes. High prevalence of icaAD was observed in device-associated strains (32.5%) mainly in orthopaedic implants, peripheral venous catheters and cardiac prosthesis. The presence of mecA gene and the occurrence of icaAD was compared in device associated isolates. Interestingly 20 MSSA strains (27.5%) possessed icaAD genes and revealed strong biofilm phenotype. Previous studies have established an important relationship between the expressions of icaAD in the absence of mecA gene.16,17 Thus, the current findings are in concordance with previous reports and it signifies the essential role of MSSA strains in device associated infections. In contrast to this observation, a recent study has shown that the excision of mecA gene in MRSA strain BH1CC, reduced the expression of FnBp proteins.18 This suggests the relation between mecA and FnBp proteins in biofilm expansion. In the present study an increased prevalence of fnbpA (19.5%) was identified in biofilm positive MRSA strains which substantiates the essential role of FnBpA in device-associated infections. Among the other ica-independent factors, the prevalence of sasG (23.5%) and clfB (18.5%) was high. Recent study has shown the mechanistic interaction of S. aureus SasG with S. epidermidis accumulation associated protein (Aap) through zinc ions.37 Thus, the presence of SasG in device-associated strains may favour S. epidermidis infections through mixed-biofilm formation on medical implants.
MTT assay revealed the increased ability of some S. aureus strains to tolerate the effect of antibiotics. But the majority of strains were susceptible to linezolid and vancomycin; they showed their highest activity on the 5th day of treatment. Among the screened isolates, vancomycin, linezolid and rifampicin were could not remove biofilms in 9 isolates. Further analysis revealed that these isolates possessed ica-dependent and independent factors. This would have favoured their increased resistance to the tested antibiotics.
In conclusion, our study on device-associated infections in two major hospitals in Mysuru, showed a high rate of multidrug resistant CA-MRSA strains. This underscores the need for routine surveillance of device-associated strains for implementing infection control strategies through proper antibiotic stewardship. Since medical implants promote biofilm formation, care must be taken while using these devices for patients’ treatment. Our study warrants the need for effective guidelines to control S. aureus infections in patients with medical implants.
FundingPradeep HP thanks Indian Council of Medical Research for the award of Senior Research Fellowship (ICMR award letter No. 80/763/2012-ECD-I dated 02.04.2013).
Conflict of interestThe authors declare no conflict of interest.
Authors are highly indebted to Dr. Wilma Ziebuhr, Institute for Molecular Infection Biology, University of Wuerzburg, Germany for providing the control strains for SCCmec types. Authors also thank Dr. Vijay Kumar and Dr. Uma Belavadi of Department of Microbiology, JSS Medical College, Mysuru and Dr. Shilpa of Department of Microbiology, K.R. Hospital, Mysuru for providing device-associated S. aureus strains.