The role of non-tuberculous mycobacteria (NTM) among cystic fibrosis (CF) patients, on occasion, remains unknown. The aim of our study is to evaluate the prevalence and clinical/microbiological characteristics of CF adult patients colonized by NTM, highlighting Mycobacterium abscessus.
MethodsA retrospective study was conducted with 92 CF adult patients: including a control group of 64 patients, not colonized by NTM, and a study group of 28 patients, colonized by NTM. We have analyzed variables such as age, F508del mutation, lung function, pancreatic involvement, auramine staining and co-colonizations between both groups.
ResultsThe prevalence of NTM found was 30.4%. The most prevalent was M. avium complex followed by M. abscessus. For M. abscessus, in the comparative study with patients colonized by other NTM, significant results were obtained for variables age.
DiscussionWe have found a high prevalence of NTM among adult patients with CF, and we associated the presence of M. asbcessus with ages less than 30 years and F508del. Due to the pathogenic role of NTM, especially M. asbcessus, multicenter studies are required within the population suffering from CF.
El papel de las micobacterias no tuberculosas (MNT) en los pacientes con fibrosis quística (FQ) está, en ocasiones, en controversia. El objetivo del trabajo es evaluar la prevalencia y las características clínicas/microbiológicas de pacientes adultos con FQ colonizados con MNT, destacando Mycobacterium abscessus.
MétodosSe ha realizado un estudio retrospectivo en 92 pacientes adultos con FQ en el que se diferenció: grupo control, 64 pacientes no colonizados por MNT, y, grupo a estudio, 28 pacientes colonizados por MNT. Se han analizado variables como la edad, mutación F508del, función pulmonar, afectación pancreática, tinción de auramina y co-colonizaciones entre ambos grupos.
ResultadosLa prevalencia de MNT encontrada ha sido 30,4%. La MNT más prevalente fue M. avium complex seguida por M. abscessus. Para M. abscessus, en el estudio comparativo con pacientes colonizados por otras MNT, se obtuvieron diferencias estadísticamente significativas en las variables de edad.
DiscusiónHemos encontrado alta prevalencia de MNT en pacientes adultos con FQ y relacionamos la aparición de M. asbcessus con edades inferiores a 30 años y F508del. Con el fin de conocer mejor el papel patógeno de las MNT, especialmente de M. asbcessus, se requieren estudios multicéntricos en población con FQ.
Cystic fibrosis (CF) is an autosomal recessive disorder and is the most common fatal genetic disease among Caucasians. In Spain, there is an estimated incidence of one in every 4500–5000 live births. It is a consequence of mutations in the gene that resides on chromosome 7q31-32. This gene encodes for a protein called the cystic fibrosis transmembrane conductance regulator (CFTR), responsible for acting as a chloride channel in exocrine tissues. Mutations in the CFTR gene lead to a lack of functionally active protein. The most common mutation is the F508del deletion, but more than 2000 possible mutations of the gene have been described, 347 causing the disease, with great variations depending on the ethnic origin and geographical location of each population.1
The defect in transport of the chloride ion leads to dehydration of respiratory, pancreatic, hepatic, intestinal and genitourinary secretions, increasing their viscosity and thickness. Among the effects, lung function becomes compromised, characterised by a decrease in forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC), in addition to pancreatic insufficiency or the development of CF-related diabetes.1
The main cause of CF-associated mortality is chronic lung disease, with a high rate of permanent colonisation and recurrent infections, the most common causative agents being Staphylococcus aureus (S. aureus), Haemophilus influenzae and Pseudomonas aeruginosa (P. aeruginosa). Colonisation/infection by nontuberculous mycobacteria (NTM) in this context is less documented due to its lower prevalence and, as a result, the clinical repercussions on CF are still not fully understood. However, as more is learned about the pathogenesis of NTM, this area is becoming increasingly important.2,3 In addition, a number of authors point to Mycobacterium abscessus (M. abscessus) as having a more pathogenic pattern, with its greater impact on lung function over time.4
The aim of this study was to determine the prevalence and clinical and microbiological characteristics of NTM-colonised/infected CF patients, and the prevalence and clinical characteristics of patients who have had a positive culture for M. abscessus, in relation to patients in whom other NTM were isolated.
MethodsThe clinical and microbiological data of adult patients treated in the dedicated CF clinic at Hospital Universitario de La Princesa in the period 2010–2017 were analysed retrospectively, establishing two groups:
- •
Study group: 28 patients selected for having at least one positive isolation of NTM. If they had more than one positive culture, the latest one was selected for the analysis.
- •
Control group: 64 patients not colonised/infected by NTM.
A sub-analysis was performed on patients with isolates positive for mycobacteria other than M. abscessus (21 patients) and those with isolates positive for M. abscessus (7 patients).
The microbiological study was carried out on the sputum samples collected from these patients. All were processed following the conventional methodology for the study of bacteria, fungi and mycobacteria. Identification of bacteria was carried out by MALDI-TOF mass spectrometry (MALDI Biotyper® System, Bruker Daltonics, Fremont, CA, USA) and MicroScan (Dade Beckman Coulter Inc., CA, USA); mycobacteria with MALDI-TOF mass spectrometry and PCR followed by reverse hybridisation (GenoType Mycobacterium CM and GenoType Mycobacterium AS, Hain Lifescience, Germany); and fungi by MALDI-TOF and microscopic identification.
The variables studied were: age at the first isolation; age at the last NTM isolation; current age of patients in the control group; gender; forced expiratory volume percentage (% FEV1); forced vital capacity percentage (% FVC); FEV1/FVC ratio; presence of pancreatic insufficiency and CF-related diabetes; determination of body mass index (BMI); presence of allergic bronchopulmonary aspergillosis (ABPA); and F508del mutation. We also analysed co-infection with S. aureus, S. aureus resistant to methicillin (MRSA), P. aeruginosa, Burkholderia spp., Aspergillus spp., Candida spp., and other fungi.
The prevalence of mycobacteria was defined as patients with at least one positive culture for NTM during the period covered by the study.
In the descriptive analysis of the quantitative variables, we used the mean and standard deviation, and for the qualitative variables, percentages. To analyse the differences between variables according to the groups, we used Student's t-test for the quantitative variables and the Chi-square test, or Fisher's test when necessary, for the qualitative variables. The maximum permissible error level was p < 0.05.
ResultsThe prevalence of NTM infection in CF patients in our unit was 30.4%.
The most common NTM isolated was Mycobacterium avium complex (MAC) in 12 patients (42.9%), followed by M. abscessus in seven patients (25%) and Mycobacterium lentiflavum in six (21.4%). Other mycobacteria isolated, in one patient each, were: Mycobacterium kansasii (3.6%); Mycobacterium gordonae (3.6%); and one patient co-colonised by Mycobacterium intracellulare and Mycobacterium chimaera (3.6%).
It was interesting that the rate of positive staining in the cases with positive isolation was 7.1%.
Tables 1 and 2 show the analysis of the variables studied in the different groups.
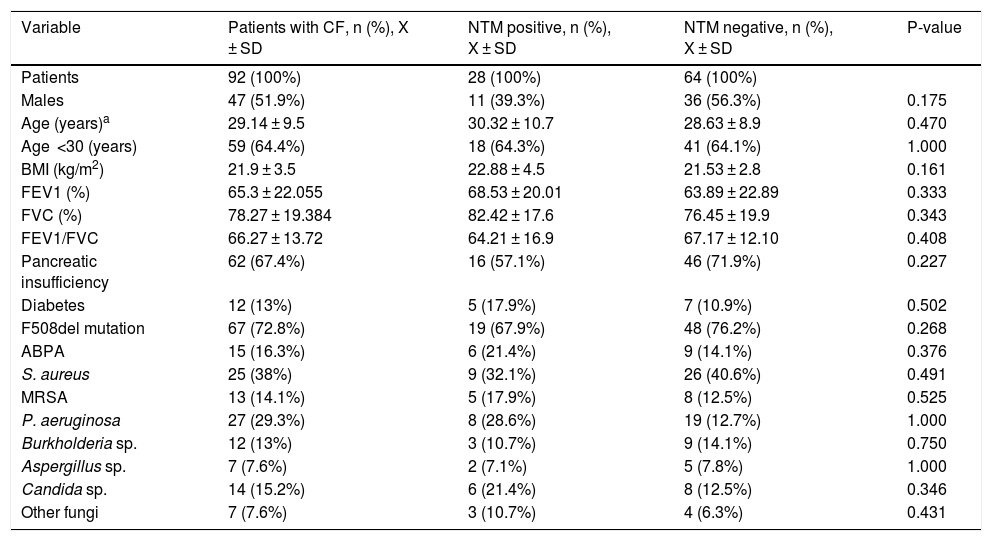
Description of the different clinical and microbiological variables in all CF patients overall and grouped into positive or negative for NTM.
| Variable | Patients with CF, n (%), X ± SD | NTM positive, n (%), X ± SD | NTM negative, n (%), X ± SD | P-value |
|---|---|---|---|---|
| Patients | 92 (100%) | 28 (100%) | 64 (100%) | |
| Males | 47 (51.9%) | 11 (39.3%) | 36 (56.3%) | 0.175 |
| Age (years)a | 29.14 ± 9.5 | 30.32 ± 10.7 | 28.63 ± 8.9 | 0.470 |
| Age <30 (years) | 59 (64.4%) | 18 (64.3%) | 41 (64.1%) | 1.000 |
| BMI (kg/m2) | 21.9 ± 3.5 | 22.88 ± 4.5 | 21.53 ± 2.8 | 0.161 |
| FEV1 (%) | 65.3 ± 22.055 | 68.53 ± 20.01 | 63.89 ± 22.89 | 0.333 |
| FVC (%) | 78.27 ± 19.384 | 82.42 ± 17.6 | 76.45 ± 19.9 | 0.343 |
| FEV1/FVC | 66.27 ± 13.72 | 64.21 ± 16.9 | 67.17 ± 12.10 | 0.408 |
| Pancreatic insufficiency | 62 (67.4%) | 16 (57.1%) | 46 (71.9%) | 0.227 |
| Diabetes | 12 (13%) | 5 (17.9%) | 7 (10.9%) | 0.502 |
| F508del mutation | 67 (72.8%) | 19 (67.9%) | 48 (76.2%) | 0.268 |
| ABPA | 15 (16.3%) | 6 (21.4%) | 9 (14.1%) | 0.376 |
| S. aureus | 25 (38%) | 9 (32.1%) | 26 (40.6%) | 0.491 |
| MRSA | 13 (14.1%) | 5 (17.9%) | 8 (12.5%) | 0.525 |
| P. aeruginosa | 27 (29.3%) | 8 (28.6%) | 19 (12.7%) | 1.000 |
| Burkholderia sp. | 12 (13%) | 3 (10.7%) | 9 (14.1%) | 0.750 |
| Aspergillus sp. | 7 (7.6%) | 2 (7.1%) | 5 (7.8%) | 1.000 |
| Candida sp. | 14 (15.2%) | 6 (21.4%) | 8 (12.5%) | 0.346 |
| Other fungi | 7 (7.6%) | 3 (10.7%) | 4 (6.3%) | 0.431 |
ABPA: allergic bronchopulmonary aspergillosis; BMI: body mass index; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; MRSA: methicillin-resistant Staphylococcus aureus.
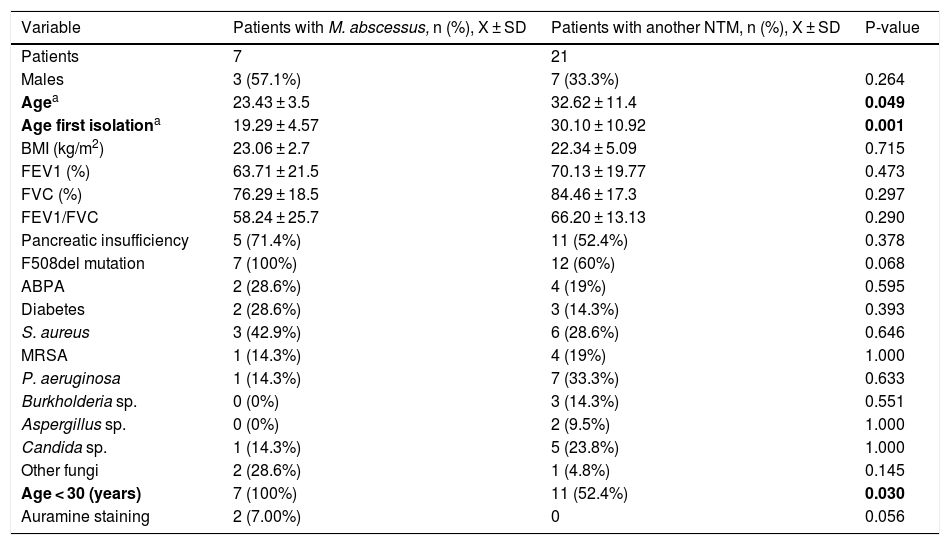
Description of the different clinical and microbiological variables in patients with M. abscessus isolation versus those with another NTM.
| Variable | Patients with M. abscessus, n (%), X ± SD | Patients with another NTM, n (%), X ± SD | P-value |
|---|---|---|---|
| Patients | 7 | 21 | |
| Males | 3 (57.1%) | 7 (33.3%) | 0.264 |
| Agea | 23.43 ± 3.5 | 32.62 ± 11.4 | 0.049 |
| Age first isolationa | 19.29 ± 4.57 | 30.10 ± 10.92 | 0.001 |
| BMI (kg/m2) | 23.06 ± 2.7 | 22.34 ± 5.09 | 0.715 |
| FEV1 (%) | 63.71 ± 21.5 | 70.13 ± 19.77 | 0.473 |
| FVC (%) | 76.29 ± 18.5 | 84.46 ± 17.3 | 0.297 |
| FEV1/FVC | 58.24 ± 25.7 | 66.20 ± 13.13 | 0.290 |
| Pancreatic insufficiency | 5 (71.4%) | 11 (52.4%) | 0.378 |
| F508del mutation | 7 (100%) | 12 (60%) | 0.068 |
| ABPA | 2 (28.6%) | 4 (19%) | 0.595 |
| Diabetes | 2 (28.6%) | 3 (14.3%) | 0.393 |
| S. aureus | 3 (42.9%) | 6 (28.6%) | 0.646 |
| MRSA | 1 (14.3%) | 4 (19%) | 1.000 |
| P. aeruginosa | 1 (14.3%) | 7 (33.3%) | 0.633 |
| Burkholderia sp. | 0 (0%) | 3 (14.3%) | 0.551 |
| Aspergillus sp. | 0 (0%) | 2 (9.5%) | 1.000 |
| Candida sp. | 1 (14.3%) | 5 (23.8%) | 1.000 |
| Other fungi | 2 (28.6%) | 1 (4.8%) | 0.145 |
| Age < 30 (years) | 7 (100%) | 11 (52.4%) | 0.030 |
| Auramine staining | 2 (7.00%) | 0 | 0.056 |
ABPA: allergic bronchopulmonary aspergillosis; BMI: body mass index; FEV1: forced expiratory volume in the first second; FVC: forced vital capacity; MRSA: methicillin-resistant Staphylococcus aureus.
In bold, statistically significant values (p-value < 0.05).
The prevalence of mycobacteria in our study (30.4%) contrasts with that reported by other studies, ranging from 3.3% to 24%.2,5,6 In our environment, this prevalence has increased since 2001.7 There may be a number of explanations for this increase: increased vigilance4; patient survival; prolonged use of inhaled antibiotic therapy3; changes in lung microbiome due to prolonged use of antimicrobials; increased awareness on the part of clinicians; and improvement in microbiological techniques for the isolation and identification of mycobacteria.
The most common NTM isolated were in line with those described in the literature,8 MAC and M. abscessus. However, the prevalences reported by Adjemian et al. – MAC 69% and M. abscessus 31% – are quite different from the 42.9% and 25% respectively found in our study. The disparity may be explained by geographical variations in NTM infection.2,5,6,8
Considering the clinical and demographic variables studied, the mean age was slightly higher in patients with a positive culture for NTM (30.3 years) compared to those with a negative culture (28.6 years), with no statistically significant differences found. The presence of mycobacteria is more common in older patients due to its opportunistic nature and for colonising lungs with previous lesions. This type of infection is also related to exposure time, which increases with the age of the patients.9 However, in the case of M. abscessus, 100% of the isolates in our study were in patients under the age of 30 (p < 0.05). This contrasts with that reported by other authors, where patients infected with MAC and M. abscessus were older when diagnosed with CF.8 Moreover, the first isolation for M. abscessus was in younger patients (p < 0.05).
The presence of NTM was not associated with worse lung function. Nevertheless, lung function was worse in patients in whom M. abscessus was isolated than in those with other NTM, although the differences were not statistically significant. It is possible that a follow-up over time would have shown us significant values, as has happened in previous studies, particularly those related to M. abscessus.4
The variables BMI, presence or absence of diabetes and pancreatic insufficiency were collected because they are associated with a worse clinical condition and can be a confounding factor when it comes to making associations. No significant results were found, however.
Although we were unable to demonstrate the association between NTM infection and the F508del mutation, it is worth mentioning that the prevalence of the F508del mutation among patients infected with NTM (67.9%) is in line with the premise that the mutation is present in approximately 70% of patients.10 Moreover, the mutation was found in 100% of the patients infected with M. abscessus and the association was almost significant. Nonetheless, more studies are needed to test the relationship, which would support the theory that infection is associated with more severe forms of CF.11
Despite the questionable associations proposed with other microorganisms,12 our study did not demonstrate any relationship between the isolation of NTM and co-infection with the pathogens studied.
The reason for having studied the clinical implications of colonisation with M. abscessus separately was that various studies have linked it to further deterioration in lung function.4 The question of whether the development of M. abscessus infection is the cause or the consequence of worsening lung function has been a matter of debate. However, both the fact that the elimination of mycobacteria leads to an improvement and the deterioration that occurs during infection support the theory that infection by M. abscessus is the cause of decreased lung function.4
This study has several limitations, as it is a retrospective study carried out in a single centre and only with an adult population. The fact that it was a cross-sectional study partially limits the variables studied, as a longitudinal follow-up of the patients, in particular their clinical outcomes and antimicrobial therapy, would have provided more information in terms of understanding the pathogenic power of these microorganisms; as well as the differentiation among the subspecies within M. abscessus.
For all these reasons, and given the pathogenic potential of NTM, especially M. abscessus, we believe a multicentre study is necessary, with a larger number of patients, including children and different geographic origins, to consolidate the results obtained in this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Fernández-Caso B, Vázquez R, Alarcón T, Girón R, López-Giménez MR, Domingo D. Prevalencia e importancia de micobacterias no tuberculosas en pacientes adultos con fibrosis quística en un hospital de Madrid. Enferm Infecc Microbiol Clin. 2020;38:323–326.