to estimate the prevalence of HCV infection in the general population of a health area through an intervention in Primary Care, differentiating between new diagnoses and infections previously diagnosed but not treated.
Methodsparticipants were selected through a risk assessment questionnaire, with all those who gave at least one affirmative answer and all those over 50 years of age undergoing a rapid test. Positive tests were confirmed in the lab by determination of anti-HCV antibodies by chemiluminescent microparticle immunoassay (CMIA) and determination of viraemia.
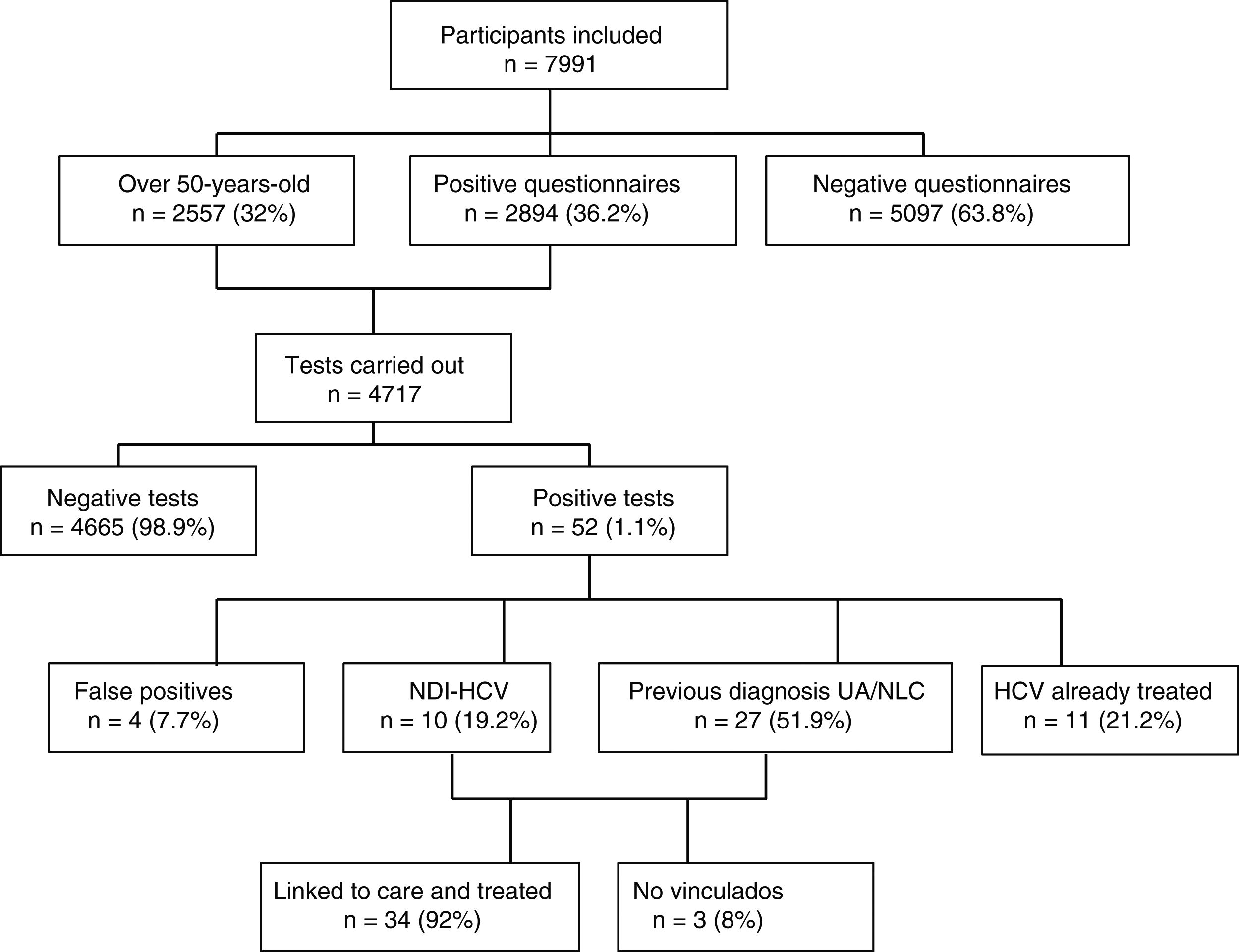
ResultsOf the 7991 participants, 36.2% presented a positive HCV risk questionnaire. 4717 tests were performed, finding an anti-HCV percentage of 0.65% in the screened population, with 0.46% of active infections. Among the individuals with a positive test result, 51.9% had a known prior diagnosis but had not received treatment, because they were not aware of it or were not linked to the health system, and 19.2% had a positive result for the first time. The prevalence of hidden infection was higher in men, those over 50 years of age and people from Eastern Europe.
ConclusionWe found a prevalence of active infections higher than recently described nationwide, and a higher percentage of newly diagnosed infections than recent similar studies in other areas. These differences justify the need to perform local assessments of the prevalence of HCV infection in each of the health areas where it is planned to implement and monitor a microelimination programme.
estimar la prevalencia de la infección por VHC en la población general de un área sanitaria a través de una intervención en Atención Primaria, diferenciando entre nuevos diagnósticos e infecciones previamente diagnosticadas pero no tratadas.
Métodosse seleccionaron participantes mediante un cuestionario de evaluación de riesgo, realizando un test rápido a todos aquellos con alguna respuesta afirmativa y a todos los mayores de 50 años. Las pruebas positivas se confirmaron en el laboratorio mediante determinación de anticuerpos frente al VHC (anti-VHC) por enzimoinmunoensayo de micropartículas quimioluminiscente (CMIA) y determinación de la viremia.
ResultadosDel total de 7991 participantes, el 36.2% presentó cuestionario de riesgo para VHC. Se realizaron 4717 test, encontrando una proporción de anti-VHC de 0.65% en la población cribada, quedando en 0.46% de infecciones activas. El 51.9% de las personas con test positivo tenían un diagnóstico previo conocido pero no habían recibido tratamiento, por no ser conscientes de ello o no encontrarse vinculados al sistema sanitario, y el 19.2% tuvo un resultado positivo por primera vez. La prevalencia de infección oculta fue mayor en hombres, mayores de 50 años, y personas procedentes de Europa del Este.
ConclusiónEncontramos una prevalencia de infecciones activas superior a la descrita recientemente a nivel nacional, y con mayor porcentaje de pacientes nuevamente diagnosticados que en trabajos similares en otras áreas. Estas diferencias justifican la necesidad de realizar evaluaciones locales de la prevalencia de infección por VHC en cada una de las áreas de salud donde se plantee implementar y monitorizar un programa de microeliminación.
Hepatitis C virus (HCV) infection is one of the leading causes of chronic liver disease.1 It is a major cause of illness in Spain,2 mainly due to the high rate of infection, particularly in the 1980s and 90s,3 and the large proportion in whom the infection becomes chronic. There have been few epidemiological studies on HCV infection in Spain. Those that do exist tend to have been on small cohorts or a selected high-risk population3–6 and do not necessarily differentiate between previously diagnosed and undiagnosed infections, all of which makes it difficult to determine the real situation of the infection today. In 2014, the prevalence of antibodies against HCV in Spain was estimated at 1.7%.7 However, in many of the studies analysed, there was no information on viraemic confirmation. Since the possibility of eradicating HCV has been opened up by the introduction of direct-acting antivirals (DAAs), there is increasing interest in having up-to-date estimates of the rates of infection in our setting. Indeed, that very fact is probably leading to dynamic changes in the current situation as regards HCV infection.8,9 The recently published results of the hepatitis C seroprevalence survey carried out by the Spanish National Health Service's Plan Estratégico para el Abordaje de la Hepatitis C (PEAHC) [Strategic Plan for Action on Hepatitis C] show a 0.85% prevalence of antibodies to HCV, with a 0.22% prevalence of active infection, in the population aged between 20 and 80.10 These overall data can differ greatly from one health area to another in Spain, so knowing these particularities is essential for the design of micro-elimination plans and for monitoring outcomes.8,9
The aim of this study is to estimate the prevalence of HCV infection in the general population of a particular health area through a Primary Care intervention using a risk assessment questionnaire, differentiating between new diagnoses and previously diagnosed infections but with patients not linked to care. We will also analyse the rate of active infections. The ultimate goal is to lay the foundations for achieving and monitoring a micro-elimination programme for HCV infection in our area.
MethodsSub-analysis of the DRIVE03 study (NCT03145753), a prospective study with 1:1 grouped randomisation carried out in four health centres (García Noblejas, Mar Báltico, Canal de Panamá and Aquitania) belonging to the Hospital Ramón y Cajal health area, in Madrid. We included people aged 18–70 who attended the health centres from November 2016 to March 2017. All participants were given a questionnaire11 assessing the risk of exposure (RE) to and indicator conditions (IC) for HIV and HCV, previously validated for HIV, and individuals with at least one affirmative answer were given a rapid test for HIV or HCV, as appropriate. Table 1 shows a summary of the questions selected from the questionnaire for assessing the risk of HCV infection, which can be consulted in full in the Banexo Appendix (see additional material). Additionally, all individuals over the age of 50 were tested for HCV, regardless of the questionnaire results. We used the anti-HCV test WB/S/P (TürkLab Laboratories, Izmir, Turkey) on a drop of blood, which provides results in approximately 15 min.12 The tests were carried out by nursing staff who were part of the research team. All individuals with a positive HCV test were informed and counselled about their results, and the coordinating staff of the Hospital Ramón y Cajal Infectious Diseases Department were notified immediately, in order to confirm the infection within 48 h in all cases where possible for the patient. In all patients with a positive result, a confirmation test was performed using a chemiluminescent microparticle enzyme immunoassay (CMIA, Architect, Abbott) and viraemia was determined (HCV core antigen, Abbott or HCV RNA, cobas® 6800 system, Roche) in the same sample by one-step diagnosis.13 Checks were made for previous positive results for HCV, taking a detailed medical history and reviewing the Electronic Medical Records, the records of the Hospital Ramón y Cajal Microbiology Laboratory and Madrid Region's HORUS platform database. All the patients who attended their appointment at Hospital Ramón y Cajal received DAA therapy, and their virologic response data were collected 12 weeks after the end of the treatment. From the introduction of DAA therapy to the start date of the study, our centre had prescribed a total of 1293 treatments for HCV infection.
Questions used to assess exposure risk and conditions indicative of HCV infection.
| Risk of exposure |
| Have you ever had/do you have a partner with HIV or HCV? |
| Have you ever had/are you having homosexual relations? |
| Did you ever have a blood transfusion before 1990? |
| Have you ever taken/do you take illegal drugs intravenously? |
| Do you think you could ever have been at risk of acquiring HIV/HCV infection by exposure? |
| Indicator conditions. Indicate if you have ever had or have: |
| A sexually transmitted infection (syphilis, gonorrhoea, Chlamydia, urethritis, herpes, lymphogranuloma) |
| Lymphoma |
| Anal/cervical cancer or dysplasia |
| Unexplained hepatitis B/C or liver disease |
| Unexplained thrombocytopenia/lymphopenia |
| Tuberculosis |
- •
HCV infection: patient with positive anti-HCV antibody result by CMIA.
- •
Known HCV infection: patient with HCV for whom there was a previous positive result for HCV documented in the Microbiology records or in the medical records, or where the patient reported it as part of their medical history. Patients with treated and cured infection were distinguished from those who were not aware of their diagnosis or were not linked to healthcare.
- •
Newly diagnosed with HCV infection: patients with HCV infection confirmed during the DRIVE03 study, in whom there was no previous record of a positive result.
- •
Active infection: patients with virus replication at the time of the assessment in the study.
- •
Estimation of occult HCV infection: estimated number of people with active HCV in our health area, applying the percentage of active infection found in our cohort over the total population for the area.
The data were entered into an Excel-2011 database (Microsoft®) using self-developed automated questionnaire reading software, and statistical analysis was performed using the Stata 15.1 package (StataCorp-LP, College Station, TX, USA). For the descriptive analysis, we used frequencies, mean with standard deviation or medians with interquartile range (IQR). We calculated the percentages of positive tests in each population subgroup (general, known HCV infection, newly diagnosed) with the 95% confidence interval. Logistic regression was used to analyse the differences in independent variables between newly diagnosed and previously diagnosed patients not linked to care. We calculated the percentage of HCV infection in each population group using the total number of participants included in the study as the denominator, considering as negative cases the individuals who had no affirmative responses in the questionnaire and were not therefore given the rapid test.
ResultsA total of 7991 participants were included in the study and completed the RE and IC questionnaire; A total of 65.9% were female and the median age was 43 (IQR 33–53). Most of the participants (40.6%) had a medium level of education, followed by 33.3% with higher education and 25.1% basic. The most common place of origin was Spain (75.5%), followed by Latin America (15.4%). In the two years prior to entering the study, 87% had made at least one visit to their general practitioner, 59% to a hospital specialist, 42% the Accident and Emergency Department and 20% the occupational health doctor.
Out of a total of 7991 participants, 2894 (36.2%) questionnaires were positive for HCV; 2261 (28.3%) due to risk of exposure, 1052 (13.2%) indicator conditions and 419 (5.2%) both. All except 94 (3%) of those with positive questionnaires had the rapid HCV test, with participant refusal being the main reason for not having the test. As 2557 participants were over the age of 50, they were also tested. Amongst those over the age of 50, there were a total of 854 (33.4%) positive questionnaires, 571 (22.3%) due to risk of exposure and 443 (17.3%), indicator conditions.
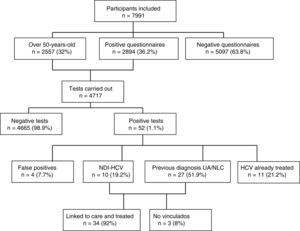
Of the 4717 tests performed, 52 (1.1%) were positive: four (7.7%) turned out to be false positives (two of which had been doubtful positives) after performing the CMIA tests at the hospital; 11 (21.2%) were people with known HCV infection and had previously been treated; 10 (19.2%) were new HCV diagnoses with active infection; and 27 (51.9%) were individuals with a previous positive HCV test, but who were either unaware of it or were not linked to health care, with the infection remaining active in 26 cases (96%) and one case (4%) found to have had spontaneous clearance. Fig. 1 shows the algorithm for HCV infection with patient flow in the DRIVE03 study.
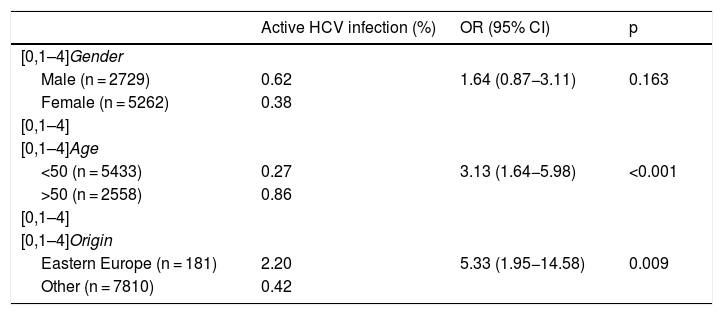
Of the total number of patients with active HCV infection, 53% were female, with a median age of 51 (IQR 49–55), A total of 85% were of Spanish nationality, 9% from Eastern Europe and 3% from Latin America. The prevalence of active infection was higher in males, in those over 50 and in people from Eastern Europe (Table 2). Out of the total, 34 (92%) were appropriately linked to care. The most common HCV genotype was 1 B (38%), followed by 1 A (27%) and 3 A (15%). The most used DAAs were Ledipasvir/Sofosbuvir (39%), Grazoprevir/Elbasvir (26%) and Sofosbuvir/Velpatasvir (21%). In all cases, a sustained virologic response was obtained at week 12.
Factors associated with the presence of active HCV infection.
| Active HCV infection (%) | OR (95% CI) | p | |
|---|---|---|---|
| [0,1–4]Gender | |||
| Male (n = 2729) | 0.62 | 1.64 (0.87−3.11) | 0.163 |
| Female (n = 5262) | 0.38 | ||
| [0,1–4] | |||
| [0,1–4]Age | |||
| <50 (n = 5433) | 0.27 | 3.13 (1.64−5.98) | <0.001 |
| >50 (n = 2558) | 0.86 | ||
| [0,1–4] | |||
| [0,1–4]Origin | |||
| Eastern Europe (n = 181) | 2.20 | 5.33 (1.95−14.58) | 0.009 |
| Other (n = 7810) | 0.42 | ||
CI: confidence interval; OR: odds ratio.
No significant differences were found in age (p = 0.589), gender (p = 0.697), degree of fibrosis (p = 0.390) or MELD score (p = 0.193) between newly diagnosed patients and previously diagnosed patients not linked to care.
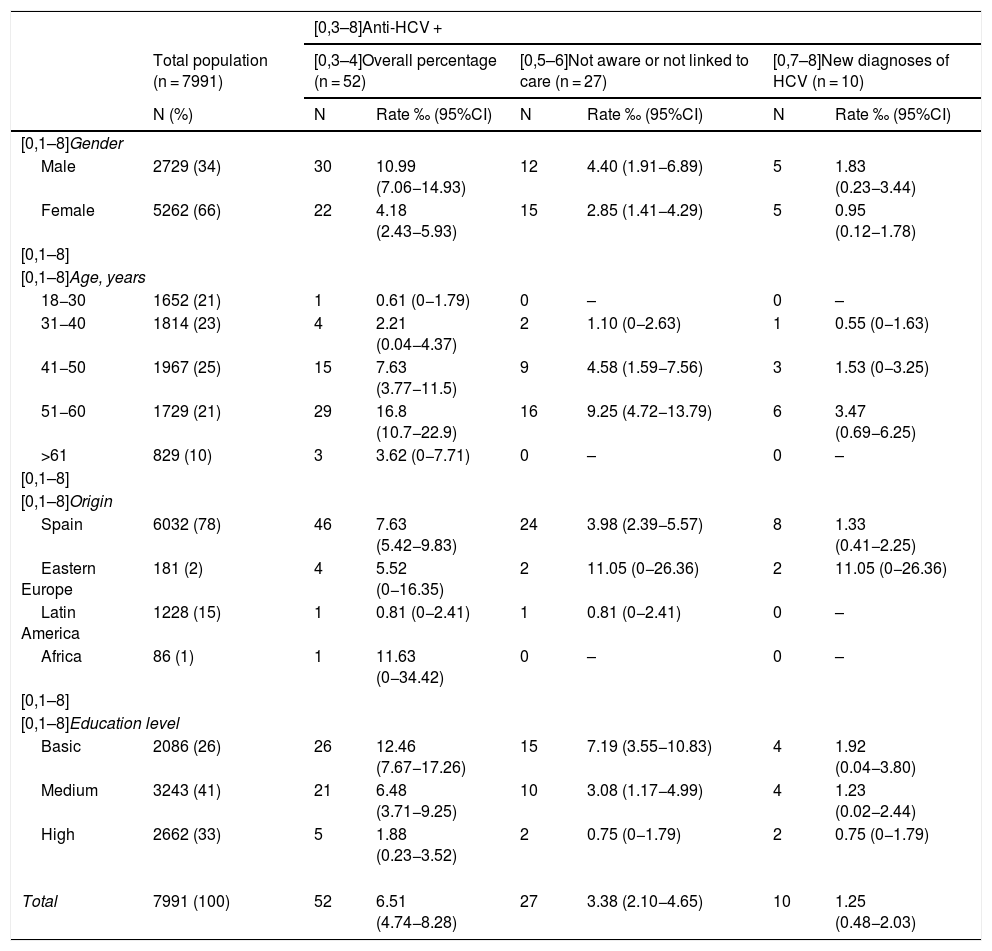
Table 3 shows the data on the proportion of HCV infection in the total population included in the study, with sub-analyses according to different population groups, differentiating between new HCV infection diagnoses and patients previously diagnosed but who were unaware or not linked to care. A higher proportion of HCV infection was found in all groups in the male population, with an overall prevalence of 1.1%, being more common in 51−60 year-olds (1.7%) and in the group with a low educational level (1.2%).
Estimation of the proportion and diagnostic rate of HCV by groups.
| [0,3–8]Anti-HCV + | |||||||
|---|---|---|---|---|---|---|---|
| Total population (n = 7991) | [0,3–4]Overall percentage (n = 52) | [0,5–6]Not aware or not linked to care (n = 27) | [0,7–8]New diagnoses of HCV (n = 10) | ||||
| N (%) | N | Rate ‰ (95%CI) | N | Rate ‰ (95%CI) | N | Rate ‰ (95%CI) | |
| [0,1–8]Gender | |||||||
| Male | 2729 (34) | 30 | 10.99 (7.06−14.93) | 12 | 4.40 (1.91−6.89) | 5 | 1.83 (0.23−3.44) |
| Female | 5262 (66) | 22 | 4.18 (2.43−5.93) | 15 | 2.85 (1.41−4.29) | 5 | 0.95 (0.12−1.78) |
| [0,1–8] | |||||||
| [0,1–8]Age, years | |||||||
| 18−30 | 1652 (21) | 1 | 0.61 (0−1.79) | 0 | – | 0 | – |
| 31−40 | 1814 (23) | 4 | 2.21 (0.04−4.37) | 2 | 1.10 (0−2.63) | 1 | 0.55 (0−1.63) |
| 41−50 | 1967 (25) | 15 | 7.63 (3.77−11.5) | 9 | 4.58 (1.59−7.56) | 3 | 1.53 (0−3.25) |
| 51−60 | 1729 (21) | 29 | 16.8 (10.7−22.9) | 16 | 9.25 (4.72−13.79) | 6 | 3.47 (0.69−6.25) |
| >61 | 829 (10) | 3 | 3.62 (0−7.71) | 0 | – | 0 | – |
| [0,1–8] | |||||||
| [0,1–8]Origin | |||||||
| Spain | 6032 (78) | 46 | 7.63 (5.42−9.83) | 24 | 3.98 (2.39−5.57) | 8 | 1.33 (0.41−2.25) |
| Eastern Europe | 181 (2) | 4 | 5.52 (0−16.35) | 2 | 11.05 (0−26.36) | 2 | 11.05 (0−26.36) |
| Latin America | 1228 (15) | 1 | 0.81 (0−2.41) | 1 | 0.81 (0−2.41) | 0 | – |
| Africa | 86 (1) | 1 | 11.63 (0−34.42) | 0 | – | 0 | – |
| [0,1–8] | |||||||
| [0,1–8]Education level | |||||||
| Basic | 2086 (26) | 26 | 12.46 (7.67−17.26) | 15 | 7.19 (3.55−10.83) | 4 | 1.92 (0.04−3.80) |
| Medium | 3243 (41) | 21 | 6.48 (3.71−9.25) | 10 | 3.08 (1.17−4.99) | 4 | 1.23 (0.02−2.44) |
| High | 2662 (33) | 5 | 1.88 (0.23−3.52) | 2 | 0.75 (0−1.79) | 2 | 0.75 (0−1.79) |
| Total | 7991 (100) | 52 | 6.51 (4.74−8.28) | 27 | 3.38 (2.10−4.65) | 10 | 1.25 (0.48−2.03) |
Applying the percentage of active infection found to the total population of 373,180 in the established age group in the health area, we calculated an estimated 1717 individuals with undiagnosed active infection in the area covered by Hospital Ramón y Cajal.
DiscussionFrom our results, the proportion of the general population screened to have anti-HCV antibodies is estimated to be 0.65%, with 0.46% estimated to have active infection, after excluding patients who had already received treatment for hepatitis C. Of the people with a positive rapid test, 51.9% had a known previous diagnosis, but had not received treatment, either because they were unaware of the diagnosis or they were not linked to the healthcare system, and 19.2% had a positive result for the first time, resulting in a prevalence 0.34% and 0.13%, respectively. These data suggest that the proportion of undiagnosed infections is low, although the figures for new diagnoses and active infections are higher than those reported in recent studies in our setting.10,14 Studies carried out in Spain in the 1990s estimated a prevalence of anti-HCV antibodies in this country in the region of 1.6% to 2.6%. However, in some cases these figures were based on a single analysis with no confirmation test, meaning that the reported rate could include false positive results, and they did not differentiate between active and non-active infections.15 Now that the prevalence of injection drug use has decreased and health controls have been established to prevent nosocomial transmission of HCV, the prevalence of HCV infection is expected to have decreased, as the deaths of people with HCV exceed new infections.16 Several seroprevalence surveys have been carried out in recent years in Spain, with very different results. In the Madrid Region in 2008–2009 the prevalence of anti-HCV antibodies in the population aged 16–80 was estimated to be 1.8% (without confirmation tests).17 Other surveys carried out in different Autonomous Regions have reported similar figures to those found in our study. In the Basque Country in 2009 a prevalence of 0.7% was found in the population under 60,18 and from 2007 to 2010 a seroprevalence of anti-HCV antibodies of 0.6% was reported in workers in Madrid and Murcia.19 As regards the proportion of HCV infections already diagnosed, the Basque Country survey found that the history of HCV infection was known in three out of four positive cases,18 and in a recent study in Navarra, this figure was 88% of the participants with a positive result.14 A recently published seroprevalence study in the general population in Spain showed a prevalence of 0.85% of antibodies against HCV, with only 0.22% of active infections.10 All these often conflicting data, in particular those on active infections, reaffirm the importance of understanding the situation in each geographic area where implementation of a micro-elimination plan for HCV infection is proposed, and of monitoring progress and outcomes.8,9
As in previous studies10,14 we found a higher proportion of males among the individuals with positive rapid test (1.1% compared to 0.4% in females). The population group with the highest proportion of people with a positive result, as already described in previous studies16 and related to the mechanisms of transmission described for Spain, was the age group of 51−60-year-olds, coinciding with the cohort of people born in the 1960s. Young people under the age of 30 show the lowest percentage of all age groups, although this figure may change in the coming years due to the increase in risk practices in the young population, especially men who have sex with men (MSM),20 who are changing the epidemiology of this infection. Eastern Europe ranks as the most common origin of patients with occult infection, consistent with the higher prevalence described in that area1 and likely the poorer access to treatment, both in their places of origin and probably also in Spain. The proportion of anti-HCV-antibody-positive individuals increases as the educational level of the screened population decreases, a finding also described in studies in other media.21 This highlights the importance of education for the population, especially in the field of sexually transmitted infections, to prevent the transmission of these infections. The above population groups with the highest prevalence of infection should also be targeted for specific micro-elimination plans, which include screening and linkage-to-care plans.
In our cohort, no significant differences were found between the demographic and clinical characteristics of newly diagnosed individuals and previously diagnosed patients, as all the diagnoses were chronic infections. It should be noted that the epidemiological changes already discussed, combined with the increase in recent years of new infections in certain population groups,20 make it essential to enhance strategies that facilitate the early diagnosis of these infections and thus prevent the progression of liver damage; A total of 95% of the patients diagnosed in our study were appropriately linked to the healthcare system and retained in care. An essential part of the screening programmes in Primary Care is the adequate linking of patients to healthcare so that they can benefit early from the specific treatment and maintain adequate follow-up, and this is known as "linkage to care". The services that perform the screening must have a well-defined communication channel for the quick and effective referral of patients, with the aim of increasing retention in care.22 Linkage to care can be suboptimal, especially in certain vulnerable groups, such as people with limited resources or injection drug users. There is some evidence to show that educational interventions for Primary Care staff can result in better linkage between patients and healthcare.23,24
The study has a series of limitations. The lack of prior validation of the questionnaire for HCV infection screening means some diagnoses may have been missed among the unscreened population. However, we assumed a high sensitivity and negative predictive value due to the open characteristics of the questions, as was confirmed in the case of HIV.11
In conclusion, these data show a similar prevalence of anti-HCV antibodies to that reported in recent studies in our setting, but with a higher prevalence of active infections than described for the whole of Spain,10 and a higher percentage of newly diagnosed patients than published recently in other areas.14 These differences highlight the need to carry out local evaluations of the prevalence of HCV in each of the health areas where implementation and monitoring of a micro-elimination programme is planned. Although these infection rates are lower than those reported in series before the development of DAAs, we must continue to be vigilant in conducting HCV screening in Primary Care, particularly in the population groups with the highest prevalence of infection (males, aged over 50, low socioeconomic level). However, it is also important to take into account how the epidemiological situation has changed over recent years in Spain.
FundingThis study was funded by three competitive grants: Instituto de Salud Carlos III [Institute of Health Carlos III] (Plan Estatal de I + D + i 2013–2016 [2013–2016 Spanish R&D&i Plan]), grants PI12-00995, PI16/00551, and Spanish Ministry of Health, Social Security and Equality, project code: EC11-144, co-financed by the European Regional Development Fund (ERDF)"A way to achieve Europe", partially funded by the project RD16/0025/0001 as part of the Plan Nacional de I + D + i [Spanish R&D&i Plan] and co-financed by Instituto de Salud Carlos III-Sub-Directorate General for Evaluation and the ERDF.
Conflicts of interestJavier Martínez-Sanz has received travel grants from ViiV Healthcare, Gilead Sciences and Janssen-Cilag. Santiago Moreno has participated in conferences and has received research grants from Abbott, Boehringer & Ingelheim, Bristol-Myers Squibb, Galaad, GlaxoSmithKline, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, Roche and Schering Plough. María Jesús Pérez Elías has done consulting work for AbbVie, Boehringer Ingelheim, ViiV Healthcare, Gilead Sciences and Janssen-Cilag; she has received clinical research grants from ViiV Healthcare, Gilead Sciences, Janssen, and Merck Sharp & Dohme, and has received financial payment as a speaker at events funded by Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, and ViiV Healthcare. The other authors have no conflicts of interest to declare.
To the DRIVE03 study group: C. Gómez-Ayerbe, M. Sánchez Conde, E. Loza, S. del Campo Terrón, A. Sánchez, A. Moreno, M. Rodríguez (Hospital Universitario Ramón y Cajal, Madrid); A. Cano, A. Fernández, M.E. Calonge, C. Santos (C.S. García Noblejas, Madrid); S. Ares (C.S. Mar Báltico, Madrid); C. Labrador (Hospital de la Princesa, Madrid); P. González (SUMMA, Madrid); L. Polo (Hospital General Universitario Gregorio Marañón, Madrid); Y. de la Fuente (C.S. Aquitania, Madrid).
Please cite this article as: Martínez-Sanz J, Muriel A, Vivancos-Gallego MJ, Galán JC, Romero B, Rodríguez-Sagrado MÁ, et al. Prevalencia de la infección por el VHC en un área sanitaria de Madrid: el primer paso para la microeliminación. Enferm Infecc Microbiol Clin. 2020;38:317–322.