Aging of people living with HIV could be related to potentially inappropiate medication prescriptions, drugs interactions and lack of drugs adherence. PIMDINAC criteria seek to jointly analyze these problems. The objective of this study is to determine the prevalence of PIMDINAC criteria in an elderly HIV population.
MethodsObservational, cross-sectional, multicenter study that included patients older than 65 years in pharmacotherapeutic follow-up between February-April 2020. The main endpoint was the percentage of PIMDINAC criteria identified in the study population.
ResultsForty-seven patientes were included, identifying total PIMDINAC in 12.5%. Non-adherence to concomitant treatment was detected in 65.6% of patients, potentially inappropiate medication in 48.9% and drugs interactions in 25.2%. The number of concomitant drugs and polypharmacy were associated with a higher appearance of PIMDINAC criteria.
ConclusionThe prevalence of PIMDINAC criteria in elderly HIV patients is high.
El envejecimiento de las poblaciones VIH podría relacionarse con prescripciones de medicación potencialmente inadecuada, interacciones medicamentosas y falta de adherencia terapéutica. Los criterios PIMDINAC buscan analizar conjuntamente estos problemas. El objetivo del estudio es determinar la prevalencia de criterios PIMDINAC en una población VIH de edad avanzada.
MétodosEstudio observacional transversal, multicéntrico que incluyó pacientes mayores de 65 años en seguimiento farmacoterapéutico entre febrero-abril 2020. La variable principal fue el porcentaje de de cumplimiento de criterios PIMDINAC.
ResultadosSe incluyeron 47 pacientes, registrándose un PIMDINAC total en un 12,5%. De forma aislada, se detectó no adherencia al tratamiento concomitante en un 65,6% de pacientes, medicación potencialmente inadecuada en un 48,9% e interacciones medicamentosas en un 25,2%. El número de fármacos y la polifarmacia se relacionaron con mayor aparición de criterios PIMDINAC.
ConclusiónLa prevalencia de criterios PIMDINAC en pacientes VIH de edad avanzada es elevada.
Recent years have witnessed the ageing of the human immunodeficiency virus (HIV)-positive population as a result of more effective, longer-lasting antiretroviral treatment (ART). It has been estimated that more than 70% of people living with HIV (PLHIV) will be over the age of 50 by 20301. In this context, the incidence of associated comorbidities and, at the same time, polypharmacy and complexity of drug treatment, is rising2. This is giving rise to a higher risk of potentially inappropriate prescriptions3, drug interactions4 and problems with non-adherence to treatment5. These issues currently represent a cause for concern in the guidelines for managing elderly PLHIV.
Each of these problems has been previously researched and measured in isolation, but no authors have characterised their joint presence in elderly PLHIV. For the purpose of overall analysis, the PIMDINAC criteria arose. These criteria refer to three components: potentially inappropriate medication (PIM), drug interactions (DI) and non-adherence to concomitant treatment (NAC).
The objective of this study was to determine the prevalence of PIMDINAC criteria (total and partial), as well as the factors associated with their presence, in a population of PLHIV over 65 years of age in real-world clinical practice.
MethodsThis observational, cross-sectional, multicentre study included all patients over 65 years of age on active ART followed up in pharmaceutical care practices at three hospitals in Andalusia, Spain, between 1 February and 30 April 2020. A cut-off age of 65 was used, since other authors6 have confirmed that, despite there being a biological basis for considering PLHIV over age 50 to be “elderly”, the prevalence of the criteria encompassed in the concept of PIMDINAC increases substantially in those over 65 years of age.
Patients participating in clinical trials and patients who did not sign the informed consent form were excluded.
Demographic variables (age, sex and date of HIV diagnosis) and clinical variables (plasma viral load, CD4 lymphocyte level, CD4/CD8 ratio and comorbidities) were collected. Drug treatment-related variables (type of ART and concomitant medication) were also collected.
The presence of polypharmacy (defined as: concomitant prescription of six or more active ingredients including ART) and major polypharmacy (11 or more active ingredients including ART), as well as the medication regimen complexity index, dichotomised as high or low, were analysed7,8.
The primary endpoint was defined as the prevalence of PIMDINAC criteria, divided into two categories: total (joint presence of PIM+DI+NAC criteria) or partial (isolated presence of one criterion or a combination of two of the criteria). Factors associated with the presence of PIMDINAC criteria were also studied.
To identify PIM, the 2014 STOPP/START criteria were used9.
The University of Liverpool database was used to identify DI between TAR and concomitant medication10. Contraindications and potential interactions were considered clinically significant. To identify DI between different non-antiretroviral drugs, the Lexicomp® tool was used11, considering DI to be grade D (potential interactions) or X (contraindications).
To assess NAC, the multi-interval coefficient of pharmacy supply in the past six months and the patients’ responses to the Adherence to Refills and Medications Scale (ARMS) were used12. A patient was considered adherent when deemed as such by both criteria. NAC based on supply record was considered to be present if patients picked up less than 90% of their drugs on time. NAC based on the ARMS was considered to apply to patients who answered “almost always” or “always” to at least two questions (in the absence of any cut-off points in the literature for this questionnaire).
Electronic medical records and the electronic prescription programme of the Servicio Andaluz de Salud [Andalusian Health Department] were used to collect data.
A descriptive statistical analysis was performed and quantitative data were summarised with measures of central tendency: mean and standard deviation, or median and interquartile range for asymmetrical data distributions. For categorical variables, frequencies and percentages were used. To evaluate the bivariate relationship between categorical variables such as the PIMDINAC criteria and other demographic and clinical variables, the chi-squared test or Fisher’s exact test was used. For quantitative variables, Student’s t test or the Mann–Whitney U test was used, depending on whether distributions were normal or not normal, respectively. Data analysis was performed with the IBM SPSS statistical package, version 20.0.
The study was approved by the Independent Ethics Committee of the Área de Gestión Sanitaria Sur de Sevilla [Southern Seville Health Management Area].
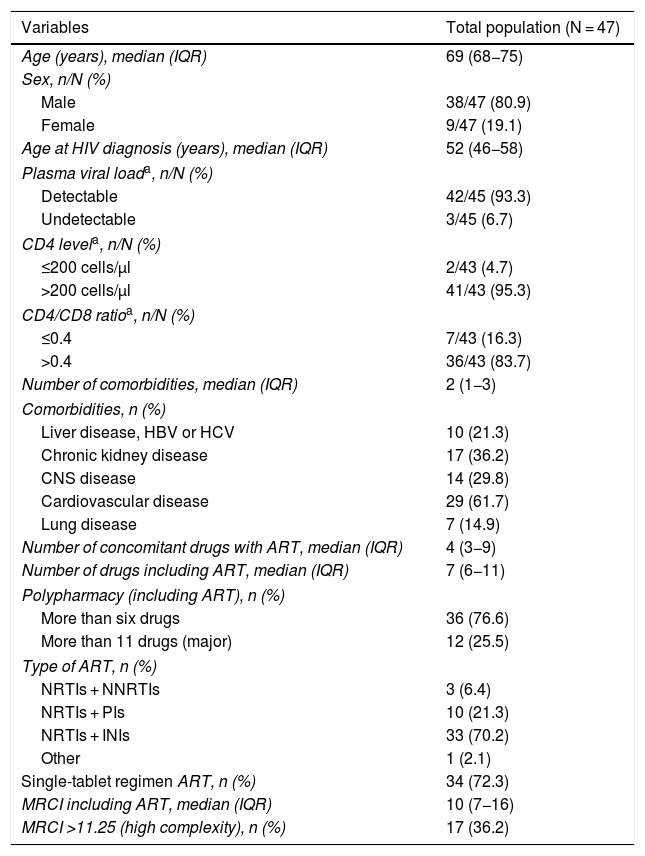
ResultsA total of 47 patients were included; their baseline characteristics are described in Table 1. Of them, 80.9% were men, and they had a median age of 69 years.
Baseline characteristics of the study patients.
| Variables | Total population (N = 47) |
|---|---|
| Age (years), median (IQR) | 69 (68−75) |
| Sex, n/N (%) | |
| Male | 38/47 (80.9) |
| Female | 9/47 (19.1) |
| Age at HIV diagnosis (years), median (IQR) | 52 (46−58) |
| Plasma viral loada, n/N (%) | |
| Detectable | 42/45 (93.3) |
| Undetectable | 3/45 (6.7) |
| CD4 levela, n/N (%) | |
| ≤200 cells/μl | 2/43 (4.7) |
| >200 cells/μl | 41/43 (95.3) |
| CD4/CD8 ratioa, n/N (%) | |
| ≤0.4 | 7/43 (16.3) |
| >0.4 | 36/43 (83.7) |
| Number of comorbidities, median (IQR) | 2 (1−3) |
| Comorbidities, n (%) | |
| Liver disease, HBV or HCV | 10 (21.3) |
| Chronic kidney disease | 17 (36.2) |
| CNS disease | 14 (29.8) |
| Cardiovascular disease | 29 (61.7) |
| Lung disease | 7 (14.9) |
| Number of concomitant drugs with ART, median (IQR) | 4 (3−9) |
| Number of drugs including ART, median (IQR) | 7 (6−11) |
| Polypharmacy (including ART), n (%) | |
| More than six drugs | 36 (76.6) |
| More than 11 drugs (major) | 12 (25.5) |
| Type of ART, n (%) | |
| NRTIs + NNRTIs | 3 (6.4) |
| NRTIs + PIs | 10 (21.3) |
| NRTIs + INIs | 33 (70.2) |
| Other | 1 (2.1) |
| Single-tablet regimen ART, n (%) | 34 (72.3) |
| MRCI including ART, median (IQR) | 10 (7−16) |
| MRCI >11.25 (high complexity), n (%) | 17 (36.2) |
ART: antiretroviral treatment; CNS: central nervous system; HBV: hepatitis B virus; HCV: hepatitis C virus; HIV: human immunodeficiency virus; INIs: integrase inhibitors; IQR: interquartile range; MRCI: medication regimen complexity index; NNRTIs: non-nucleoside reverse-transcriptase inhibitors; NRTIs: nucleoside reverse-transcriptase inhibitors; PIs: protease inhibitors.
The presence of total PIMDINAC criteria was detected in 12.5% of the patients.
NAC was identified in 65.6% of the patients (n/N = 21/32), PIM in 48.9% (n/N = 23/47) and DI in 25.2% (n/N = 12/47). In addition, 34.4% of the patients simultaneously presented PIM + NAC (n/N = 11/32), 18.8% DI + NAC (n/N = 6/32) and 17.0% PIM + DI (n/N = 8/47).
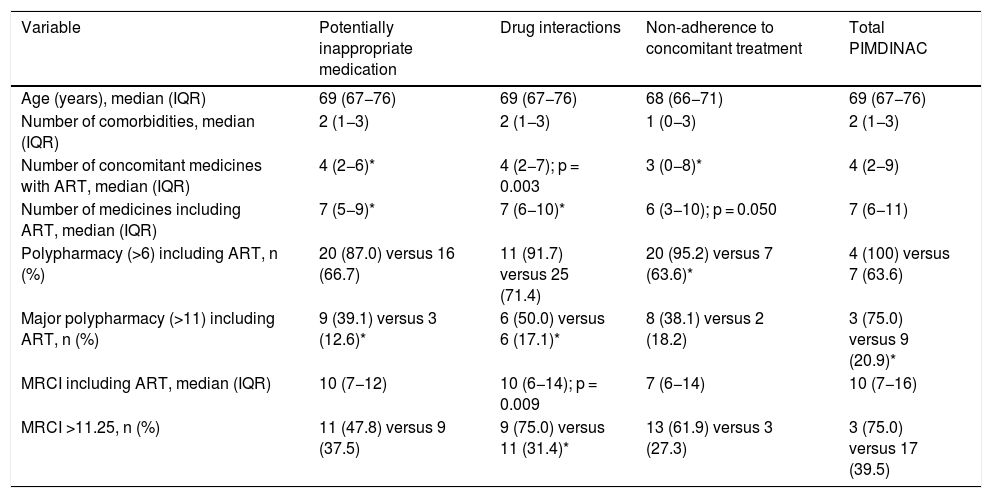
The analysis of the factors associated with the presence of PIMDINAC criteria is detailed in Table 2.
Bivariate analysis of factors associated with the presence of PIMDINAC criteria.
| Variable | Potentially inappropriate medication | Drug interactions | Non-adherence to concomitant treatment | Total PIMDINAC |
|---|---|---|---|---|
| Age (years), median (IQR) | 69 (67−76) | 69 (67−76) | 68 (66−71) | 69 (67−76) |
| Number of comorbidities, median (IQR) | 2 (1−3) | 2 (1−3) | 1 (0−3) | 2 (1−3) |
| Number of concomitant medicines with ART, median (IQR) | 4 (2−6)* | 4 (2−7); p = 0.003 | 3 (0−8)* | 4 (2−9) |
| Number of medicines including ART, median (IQR) | 7 (5−9)* | 7 (6−10)* | 6 (3−10); p = 0.050 | 7 (6−11) |
| Polypharmacy (>6) including ART, n (%) | 20 (87.0) versus 16 (66.7) | 11 (91.7) versus 25 (71.4) | 20 (95.2) versus 7 (63.6)* | 4 (100) versus 7 (63.6) |
| Major polypharmacy (>11) including ART, n (%) | 9 (39.1) versus 3 (12.6)* | 6 (50.0) versus 6 (17.1)* | 8 (38.1) versus 2 (18.2) | 3 (75.0) versus 9 (20.9)* |
| MRCI including ART, median (IQR) | 10 (7−12) | 10 (6−14); p = 0.009 | 7 (6−14) | 10 (7−16) |
| MRCI >11.25, n (%) | 11 (47.8) versus 9 (37.5) | 9 (75.0) versus 11 (31.4)* | 13 (61.9) versus 3 (27.3) | 3 (75.0) versus 17 (39.5) |
ART: antiretroviral treatment; IQR: interquartile range; MRCI: medication regimen complexity index.
PIMDINAC criteria were seen in a significant percentage of patients and at higher rates when the different criteria were analysed in isolation. Major polypharmacy was associated with the presence of total PIMDINAC.
The most commonly detected PIMDINAC component was NAC. This might have been due to the high rate of polypharmacy present in our population. A statistically significant relationship was seen between a larger number of prescribed medicines and the presence of polypharmacy with NAC. Other authors have previously reported the same findings13. Similarly, a high percentage of patients (36.2%) had a high medication regimen complexity index, which also has previously been linked to non-adherence in HIV-infected patients5, although this study did not find a statistically significant relationship.
In addition, PIM was observed in 48.9% of patients; this rate was similar to that reported in a recent study conducted in another cohort of elderly PLHIV, in which 49.5% of patients had at least one prescription medication that had to be suspended3. In this vein, our study appeared to show a relationship between the number of prescription medications and the prescription of PIM.
Finally, 25.2% of the patients had clinically significant DI, and furthermore a positive relationship was observed between this variable and a higher medication regimen complexity index.
The association between polypharmacy, DI and PIM has already been established by other authors; thus the concept of an “iatrogenic triad” has come into use in recent years6,14. However, no authors have integrated this concept with adherence problems. This study sought to integrate all criteria that could result in safety problems in patients, and NAC was the most commonly detected criterion. Therefore, we believe that the PIMDINAC criteria could constitute a new concept that is more complete than the previous one.
In light of our findings and those of other authors15, optimisation of drug treatment in PLHIV takes on particular importance; deprescription of concomitant medication may be a potentially useful strategy for multidisciplinary teams who provide healthcare to these patients.
The main limitations of this study were its observational design and its limited sample size. However, given the multicentre design of the study, its sample may represent the characteristics of the population cared for at present and, therefore, it may be possible to extrapolate the study’s findings to other settings.
Given the characteristics of the patients included, the prevalence of PIMDINAC could be expected to rise as the mean age of the PLHIV increases. Thus a multidisciplinary approach to these problems is essential to achieve optimisation of drug treatment in this population.
Future lines of research that include larger numbers of patients would also enable determination of the relationship of between the onset of these problems and the health outcomes of the patients. This may be beneficial for firmly establishing the practical utility of these criteria in healthcare practice.
Based on the characteristics of the study, which can be considered a proof of concept, it can be concluded that the prevalence of PIMDINAC criteria in PLHIV over 65 years of age is high and requires more attention in order to achieve optimisation of drug treatment in these patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Díaz-Acedo R, Soriano-Martinez M, Gutiérrez-Pizarraya A, Fernandez-Gonzalez-Caballos JA, Raya-Siles M, Morillo-Verdugo R. Prevalencia y factores asociados con la presencia de criterios PIMDINAC en pacientes VIH de edad avanzada. Enferm Infecc Microbiol Clin. 2022;40:258–261.