Survival in people living with HIV (PLWH) has increased and thus people are aging with HIV, increasing the frequency of multimorbidity and polypharmacy. This cross-sectional study was conducted to evaluate the prevalence of polypharmacy among PLWH who were on antiretroviral treatment and were followed in an outpatient setting by the pharmacy department of several hospitals across Spain. In addition, we aimed to evaluate factors associated with polypharmacy and treatment complexity among this population.
Material and methodsWe recorded information on demographic data, data on disease control including viral load and CD4 count at the time of inclusion, comorbidities, pharmacologic treatment and drugs interactions. Polypharmacy was defined as the use of 6 or more different drugs, including antiretroviral medication; major polypharmacy was defined as the use of ≥11 different drugs.
ResultsOverall, 1225 PLWH were eligible in the study. The median (IQR) age was 49 (40–54). Comorbidities were present in 819 (67%) PLWH and 571 (47%) had two or more comorbidities. Overall, 397 (32.4%, 95% CI 29.8–34.9) PLWH met the criteria for polypharmacy, and 67 (5.5%, 95% CI, 4.2–6.7) had major polypharmacy. Several factors were associated with polypharmacy such as type of antiretroviral treatment, presence of potential interactions, the use of several types of medications and the number of comorbidities. Treatment complexity was also a factor strongly associated with polypharmacy; for each point increase in the medication regimen complexity index (MRCI), the likelihood of polypharmacy increased 2.3-fold.
ConclusionsPolypharmacy is frequent among PLWH in Spain and contributes to a relevant extent to treatment complexity.
La supervivencia de las personas con infección por el VIH ha aumentado notablemente en los últimos años incrementado la edad de estos sujetos. Ello se asocia con una mayor presencia de multimorbilidad y polifarmacia. El objetivo de este estudio es evaluar la prevalencia de la polifarmacia en pacientes VIH+ con tratamiento antirretroviral activo seguidos en las consultas externas de los servicios de farmacia hospitalaria en toda España. Adicionalmente, analizar los factores asociados a polifarmacia y a la complejidad farmacoterapéutica en esta población.
Material y métodosEstudio multicéntrico, transversal. Se recogieron variables demográficas, variables relacionadas con el control de la enfermedad como la carga viral y los linfocitos CD4, las comorbilidades, el tratamiento farmacológico completo del paciente y la presencia de interacciones. La polifarmacia se definió como el uso de al menos 6 fármacos incluyendo el TAR. Se definió polifarmacia mayor como la toma de más de 11 fármacos diferentes. Se midió la complejidad farmacoterapéutica por la escala de valoración Medication Regimen Complexity Index (MRCI).
ResultadosSe incluyeron 1.225 pacientes. La mediana (RIQ) de edad fue de 49 años (40-54). En total 819 (67,0%) pacientes presentaban al menos una comorbilidad en el momento del estudio, teniendo 2 o más comorbilidades, el 47,0% de los mismos. Un total de 397 (32,4%; IC 95%: 29,8-34,9) pacientes cumplieron los criterios de polifarmacia y 67 (5,5%; IC 95%: 4,2-6,7) los de polifarmacia mayor. Los factores asociados con la polifarmacia fueron: el tratamiento antirretroviral, la presencia de interacciones potenciales, el uso de diferentes tipos de fármacos y el número de comorbilidades. La complejidad farmacoterapéutica se asoció de forma importante con la presencia de polifarmacia, incrementándose su probabilidad de aparición entre 2 y 3 veces por cada incremento en un punto en su escala de valoración.
ConclusiónLa polifarmacia es frecuente y se asocia altamente a la complejidad farmacoterapéutica en pacientes con infección por VIH en España.
The marked improvement in the potency, side effects and ease-of-use of antiretroviral therapy (ART) as well as the improvement in the management of opportunistic infections and comorbidities in recent years has led to increased survival in people living with HIV (PLWH).1 As a consequence, these persons are aging,2,3 and consequently, suffering from many of the diseases associated with aging (cardiovascular disease, chronic kidney disease, etc.).4 This situation has led to an increased frequency of multimorbidity and polypharmacy in a substantial proportion of PLWH. So, in the US, it has been estimated that multimorbidity has increased between 2000 and 2009 from 8% to 22%, respectively.5 Likewise, the prevalence of polypharmacy in PLWH is increasing6–12 in the US from 16% in 2006 to 35% in 20108 and among those aged 50 years or older in Switzerland from 38% in 2004 to 47% in 2016.6 Polypharmacy in PLWH has been associated with the presence of serious adverse events,12 an increased risk of drug-drug interactions,13,14 ART discontinuation,9 and an increased risk of hospitalization and mortality.11 Polypharmacy has also been associated with an increased complexity of treatment regimens, which, in turn, is associated with reduced treatment adherence.15,16
Despite the increasing prevalence and clinical relevance of polypharmacy in PLWH, the role of treatment complexity still hasn’t been enough research. For this reason we conducted a cross-sectional study to evaluate the prevalence of polypharmacy among PLWH who were on ART and were followed in an outpatient setting by the pharmacy department of several hospitals across Spain. In addition, factors associated with polypharmacy and treatment complexity among this population was evaluated.
Materials and methodsStudy design and subjectsThis was a cross-sectional study conducted in the pharmacy departments of 81 hospitals across all regions of Spain. The study was performed on a single day in February 2017. The study was approved by the Ethics Committee “Comité Ético de Investigación del Sur de Sevilla” (Sevilla, Spain).
PLWH over 18 years who were on ART and visited the pharmacy department on the day of the study were included. PLWH were excluded if they were hospitalized, were participating in a clinical trial, or did not give their written informed consent.
Outcomes measuresAll information was recorded from the clinical history and other electronic records except for the evaluation of compliance that was performed by patient interview. We recorded information about demographic data, disease control including viral load and CD4 count at the time of inclusion, comorbidities, pharmacologic treatment and drugs interactions. PLWH who were on ART and visited the pharmacy department on the day of the study were included. The SMAQ is a questionnaire based on the Morisky-Green-Levine questionnaire and developed in our setting to evaluate adherence in PLWH; it consists of 6 items that evaluate forgetfulness, routine, adverse events and missing doses.17 The Morisky-Green-Levine questionnaire consisted of four items that evaluate forgetfulness, routine, adverse events and, in contrast to the SMAQ, evaluates the impact of feeling better and does not evaluate missing doses18; we used the Spanish validated version.19 The MRCI is a validated 65-item tool that evaluates treatment regimen complexity based on the number of medications, dosage form, dosage frequency, and additional or special instructions; the MRCI index score ranges from 1.5 (for someone taking a single tablet or capsule taken once a day) to an undefined maximum since the score increases with the number of medications; greater scores indicate higher complexity.20
Adherence was quantified as the proportion of days covered (PDC) according to pharmacy records. The PDC was based on the filled e-prescriptions during the 6 months prior to the study. To calculate the PDC, we estimated the total days of supplies from the first refill to the last refill during the 6-month observation period divided by the total days of the treatment interval; the treatment interval was defined as the time elapsed from the date of the first refilled prescription to the end of the observation period. The resulting figure was multiplied by 100 to estimate the PDC. A PLWH was considered adherent to ART if, according to hospital pharmacy records, the PDC was>95% and the PLWH was not positive on the SMAQ (where positive means that there was a positive response to any of the qualitative questions of the SMAQ), no more than two doses were missed over the past week, or they had fewer than 2 days of total nonmedication during the past 3 months. To evaluate adherence to concomitant medication, we only considered disease-modifying medications (e.g., treatment for diabetes, cardiovascular disease, etc.) but not symptomatic treatments (e.g., analgesics, medications for gastroesophageal reflux, etc.). A PLWH was considered adherent to concomitant medication if, according to electronic pharmacy dispensing records, the PDC was>90% and the Morisky-Green-Levine questionnaire score was 4. Polypharmacy was defined as the use of 6 or more different drugs, including antiretroviral medication; major polypharmacy was restricted to the use of ≥11 different drugs. To describe the patterns of polypharmacy, the categorization proposed by Calderón-Larrañaga et al.21 was employed; it classified the patterns depending on the type of disease they were intended to treat: cardiovascular, depression-anxiety, acute respiratory infection, chronic pulmonary disease, rhinitis-asthma, pain and menopause. After categorizing a drug according to the anatomical therapeutic chemical classification system up to the first three levels, a patient was categorized to a specific pattern when he/she was dispensed at least three drugs included in the pattern.
Statistical analysisThe number of patients on ART in Spain according to the report of the National AIDS Plan (2016) was 117.944 in 2016.22 To estimate the prevalence of polypharmacy in the HIV population with a confidence level of 95% and a precision of 5%, we started from an estimated polypharmacy prevalence of 30% with an expected loss of 25%, and we determined that we needed to analyze a total sample of 403 patients.
The quantitative variables were expressed as the means and standard deviations, or medians and interquartile ranges in the case of asymmetry, and qualitative variables were expressed as percentages. The comparison of quantitative variables between two groups was performed with Student's t test or the Mann–Whitney U test in cases of nonnormality for independent samples. Significant differences were quantified with 95% confidence intervals. The analysis of the associations between qualitative variables was carried out by applying the chi-Square test or the Monte Carlo and exact test methods to contingency tables. To find factors associated with polypharmacy, a multivariate binary logistic regression model was performed after the corresponding univariate analysis that identified the variables associated with polypharmacy at the 5% significance level. Afterwards, all the variables with significance level<25% were considered and introduced in the multivariate model for the last selection of the variable subset that profiles the occurrence of polypharmacy. The discriminatory capacity of the model was analyzed through the AUC of the ROC curve, which were equivalent to the value of Harrell's C statistic and the measure of the internal validity of the model. Likewise, the model was calibrated with the Hosmer and Lemeshow goodness of fit tests, which analyzed the agreement between the observed results and those predicted by the model.
The analysis of the data was performed with IBM SPSS 25.0 statistical software (IBM Corp., Armonk, NY, USA).
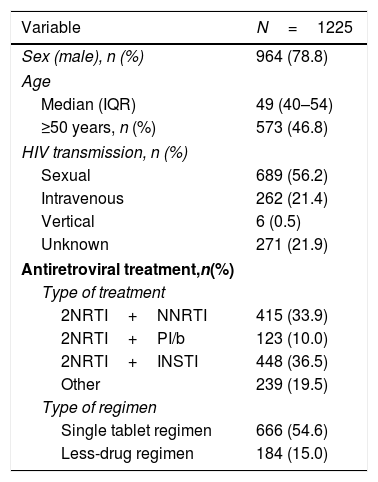
ResultsPatient disposition and characteristicsA total of 1225 PLWH were included in the analyses. They were predominantly male (79%), and 47% were 50 years or older. Most PLWH had an undetectable viral load, and over half were receiving a single tablet regimen (Table 1).
Demographic and clinical characteristics of people living with HIV in the POINT cohort.
| Variable | N=1225 |
|---|---|
| Sex (male), n (%) | 964 (78.8) |
| Age | |
| Median (IQR) | 49 (40–54) |
| ≥50 years, n (%) | 573 (46.8) |
| HIV transmission, n (%) | |
| Sexual | 689 (56.2) |
| Intravenous | 262 (21.4) |
| Vertical | 6 (0.5) |
| Unknown | 271 (21.9) |
| Antiretroviral treatment,n(%) | |
| Type of treatment | |
| 2NRTI+NNRTI | 415 (33.9) |
| 2NRTI+PI/b | 123 (10.0) |
| 2NRTI+INSTI | 448 (36.5) |
| Other | 239 (19.5) |
| Type of regimen | |
| Single tablet regimen | 666 (54.6) |
| Less-drug regimen | 184 (15.0) |
INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitors; PI/b, protease inhibitor booste.
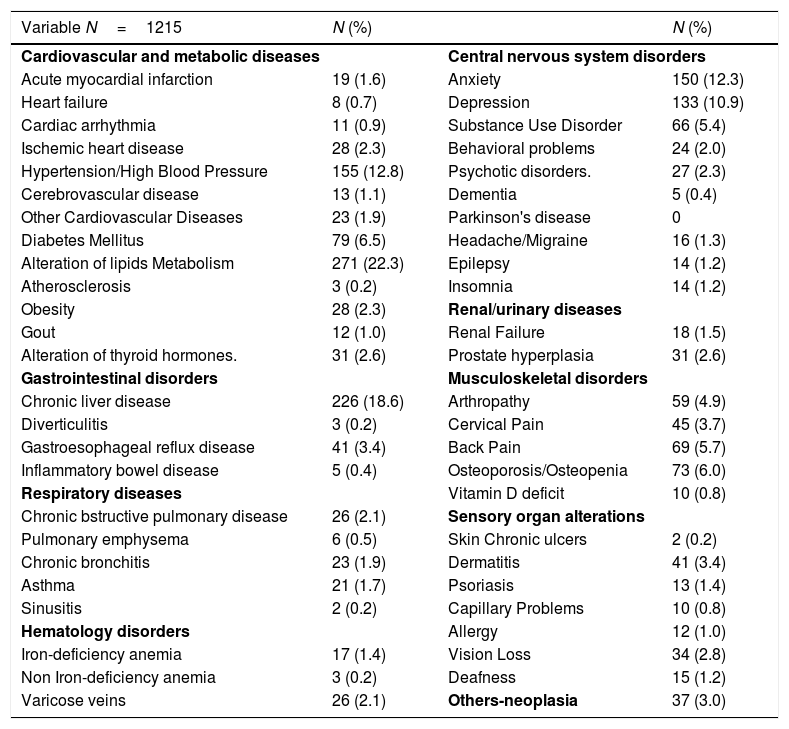
Comorbidities were present in 819 (67%), 571 (47%) had two or more comorbidities. The most frequent comorbidities were central nervous system disorders (41.1%), liver disease (18.6%), hypertension or cardiovascular disease (17.5%) and respiratory disease (6.1%). These comorbidities are detailed in Table 2.
Comorbidities of people living with HIV in the POINT cohort.
| Variable N=1215 | N (%) | N (%) | |
|---|---|---|---|
| Cardiovascular and metabolic diseases | Central nervous system disorders | ||
| Acute myocardial infarction | 19 (1.6) | Anxiety | 150 (12.3) |
| Heart failure | 8 (0.7) | Depression | 133 (10.9) |
| Cardiac arrhythmia | 11 (0.9) | Substance Use Disorder | 66 (5.4) |
| Ischemic heart disease | 28 (2.3) | Behavioral problems | 24 (2.0) |
| Hypertension/High Blood Pressure | 155 (12.8) | Psychotic disorders. | 27 (2.3) |
| Cerebrovascular disease | 13 (1.1) | Dementia | 5 (0.4) |
| Other Cardiovascular Diseases | 23 (1.9) | Parkinson's disease | 0 |
| Diabetes Mellitus | 79 (6.5) | Headache/Migraine | 16 (1.3) |
| Alteration of lipids Metabolism | 271 (22.3) | Epilepsy | 14 (1.2) |
| Atherosclerosis | 3 (0.2) | Insomnia | 14 (1.2) |
| Obesity | 28 (2.3) | Renal/urinary diseases | |
| Gout | 12 (1.0) | Renal Failure | 18 (1.5) |
| Alteration of thyroid hormones. | 31 (2.6) | Prostate hyperplasia | 31 (2.6) |
| Gastrointestinal disorders | Musculoskeletal disorders | ||
| Chronic liver disease | 226 (18.6) | Arthropathy | 59 (4.9) |
| Diverticulitis | 3 (0.2) | Cervical Pain | 45 (3.7) |
| Gastroesophageal reflux disease | 41 (3.4) | Back Pain | 69 (5.7) |
| Inflammatory bowel disease | 5 (0.4) | Osteoporosis/Osteopenia | 73 (6.0) |
| Respiratory diseases | Vitamin D deficit | 10 (0.8) | |
| Chronic bstructive pulmonary disease | 26 (2.1) | Sensory organ alterations | |
| Pulmonary emphysema | 6 (0.5) | Skin Chronic ulcers | 2 (0.2) |
| Chronic bronchitis | 23 (1.9) | Dermatitis | 41 (3.4) |
| Asthma | 21 (1.7) | Psoriasis | 13 (1.4) |
| Sinusitis | 2 (0.2) | Capillary Problems | 10 (0.8) |
| Hematology disorders | Allergy | 12 (1.0) | |
| Iron-deficiency anemia | 17 (1.4) | Vision Loss | 34 (2.8) |
| Non Iron-deficiency anemia | 3 (0.2) | Deafness | 15 (1.2) |
| Varicose veins | 26 (2.1) | Others-neoplasia | 37 (3.0) |
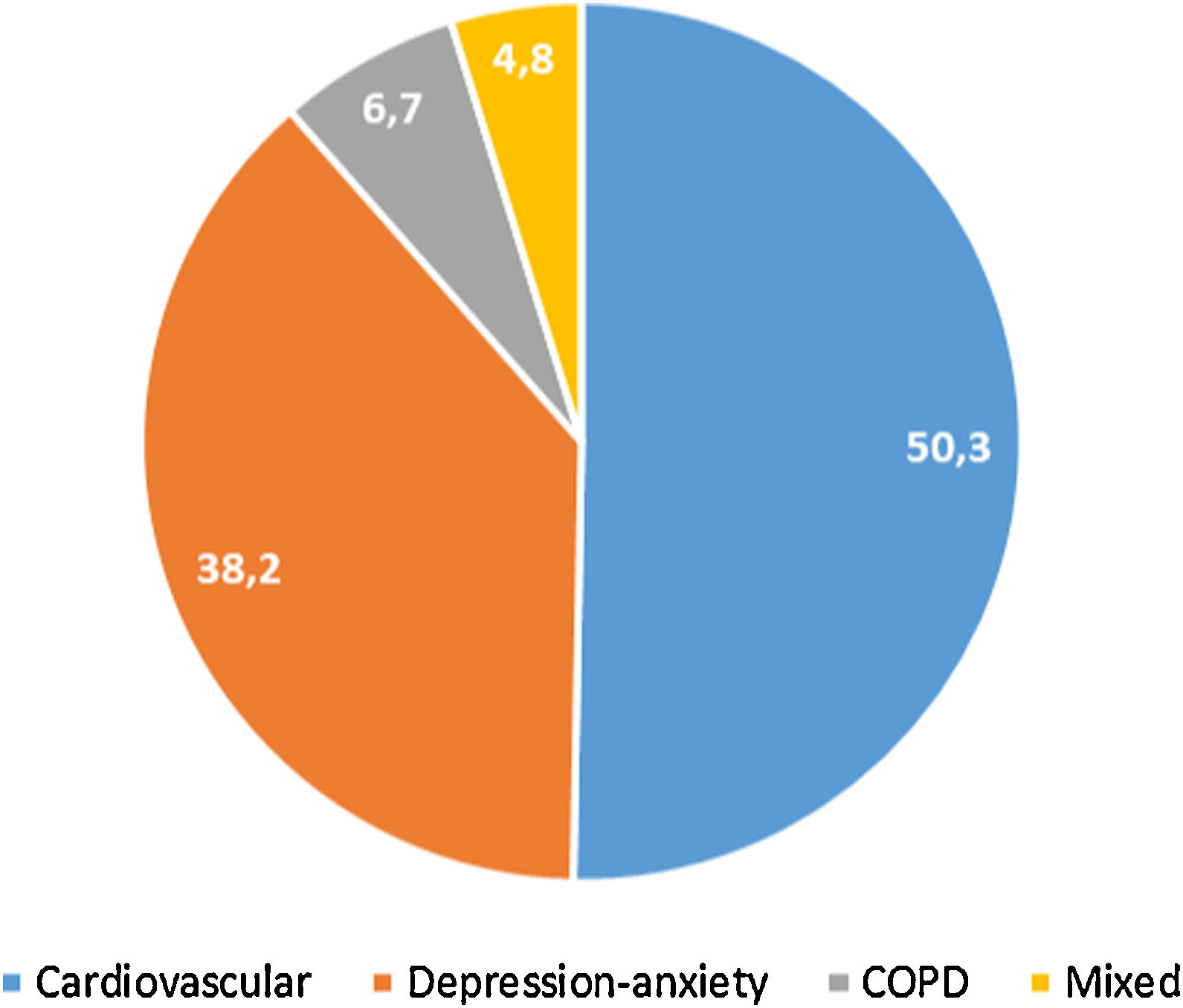
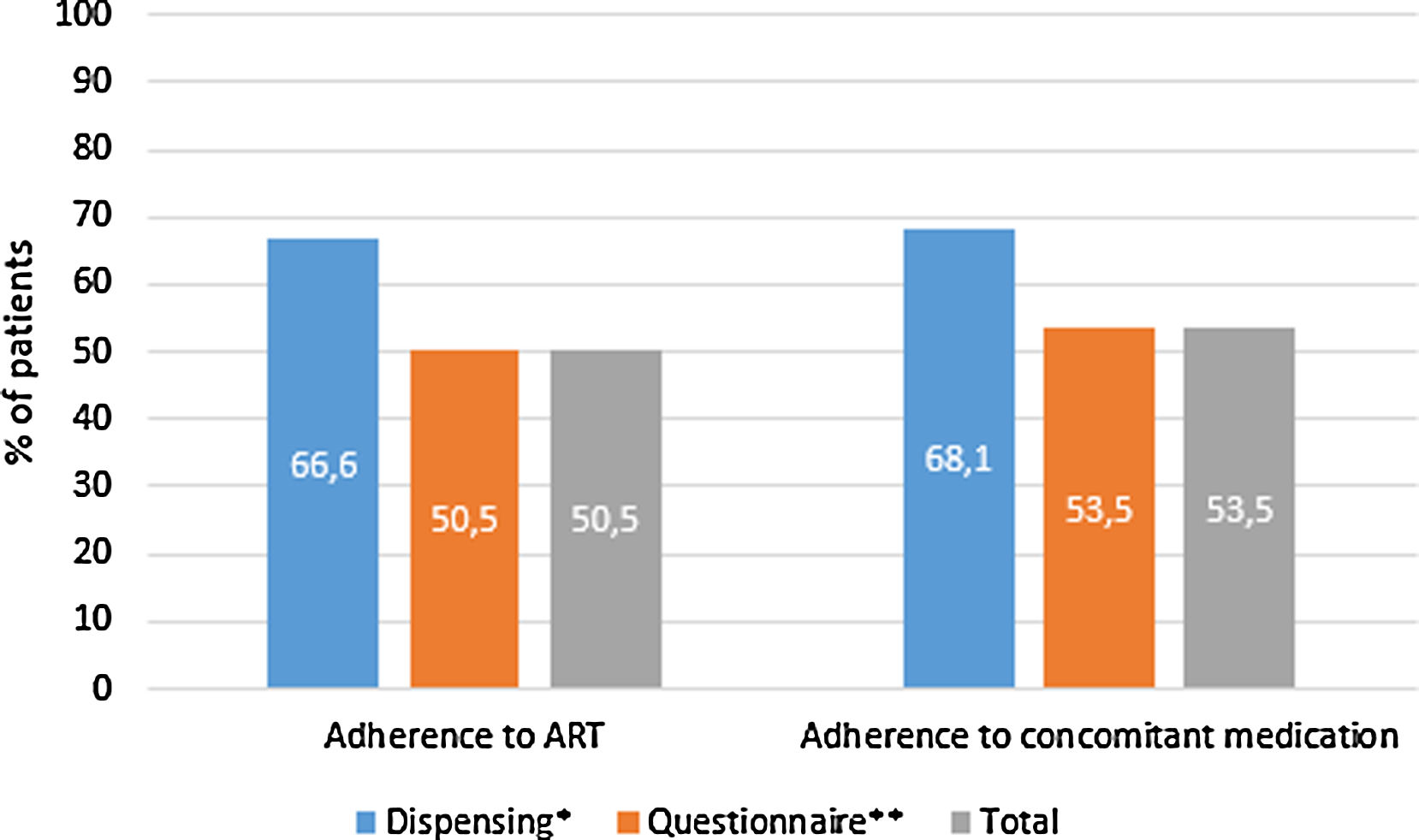
397 (32.4%, IC95% 29.8–34.9) met the criteria for polypharmacy, and 67 (5.5%, IC95% 4.2–6.7) had major polypharmacy. Among the 202 evaluable patients for this outcome, the most frequent patterns of polypharmacy were cardiovascular and depression-anxiety (Fig. 1). The median (IQR) of concomitant medications was 1 (0–3), and 509 (42%) were receiving 2 or more concomitant medications. In these same way, MRCI scores was of 3 (3–4) for the ART, 3 (0–7) for concomitant medication, and 6 (3–11) for the total score. Approximately half of the PLWH were considered adherent to the antiretroviral regimen or concomitant medications (Fig. 2).
Patterns of polypharmacy in people living with HIV in the POINT cohort. Patterns were categorized according to the type of disease they were intended to treat: cardiovascular, depression-anxiety, acute respiratory infection, chronic pulmonary disease, rhinitis-asthma, pain and menopause. The mixed pattern consisted of patients who were dispensed drugs belonging to 2 or more categories.
Treatment adherence in people living with HIV in the POINT cohort. ART, antiretroviral therapy. Adherence according to dispensing information: >95% for the ART therapy and >90% for concomitant medication. Adherence according to the questionnaire: for ART therapy, the subject was not positive in the SMAQ (where positive means that there was a positive response to all of the qualitative questions of the SMAQ), no more than two doses were missed over the past week, or there were no more than 2 days of total nonmedication during the past 3 months; for the concomitant medication, a score of 4 in the Morisky-Green-Levine questionnaire. Total adherence: the subject had to meet both criteria.
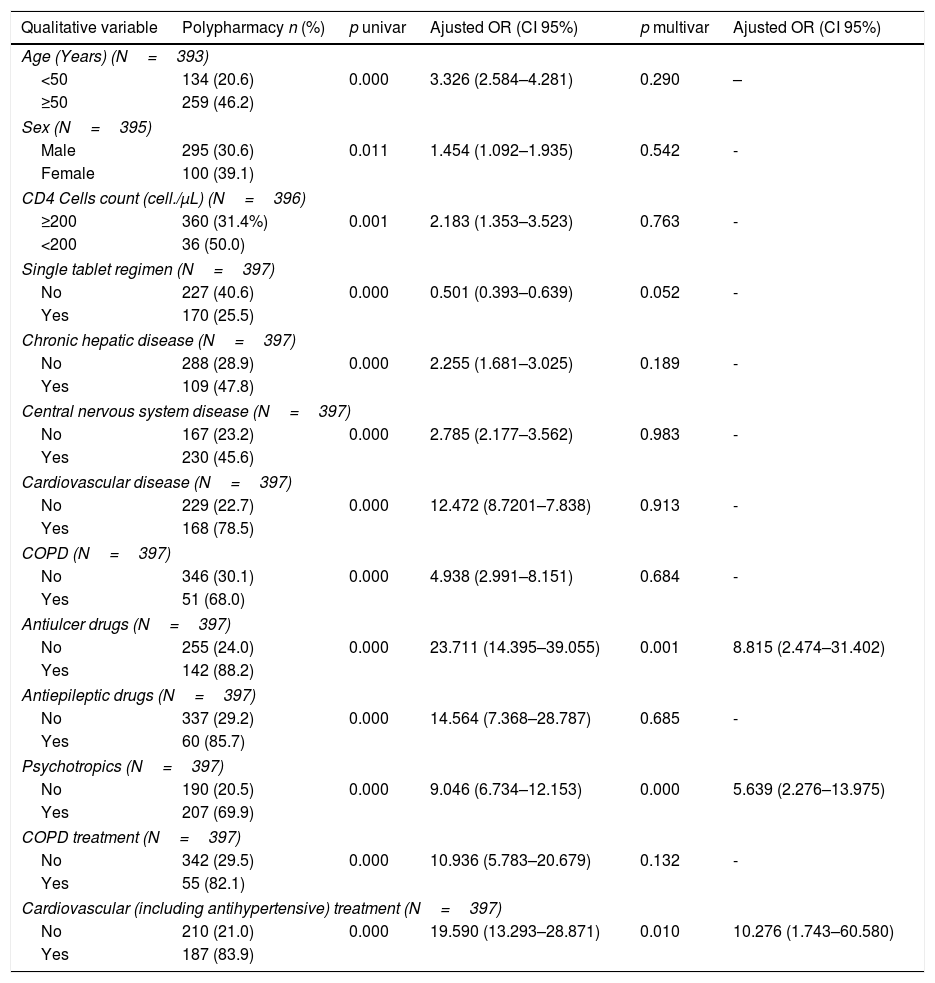
The factors associated with the presence of polypharmacy according to the univariate and multivariate analysis are presented in Table 3. The sensitivity and specificity of the equation derived from the model were 92.7% and 96.7%, respectively, predicting the presence of polypharmacy (area under the curve=0.987, 95% CI=0.980–0.994). Treatment complexity was a factor strongly associated with polypharmacy; for each point increase in the MRCI, the likelihood of polypharmacy increased 2.3-fold (Table 3).
Univariate and multivariate analysis of the factors associated with polypharmacy in people living with HIV.
| Qualitative variable | Polypharmacy n (%) | p univar | Ajusted OR (CI 95%) | p multivar | Ajusted OR (CI 95%) |
|---|---|---|---|---|---|
| Age (Years) (N=393) | |||||
| <50 | 134 (20.6) | 0.000 | 3.326 (2.584–4.281) | 0.290 | – |
| ≥50 | 259 (46.2) | ||||
| Sex (N=395) | |||||
| Male | 295 (30.6) | 0.011 | 1.454 (1.092–1.935) | 0.542 | - |
| Female | 100 (39.1) | ||||
| CD4 Cells count (cell./μL) (N=396) | |||||
| ≥200 | 360 (31.4%) | 0.001 | 2.183 (1.353–3.523) | 0.763 | - |
| <200 | 36 (50.0) | ||||
| Single tablet regimen (N=397) | |||||
| No | 227 (40.6) | 0.000 | 0.501 (0.393–0.639) | 0.052 | - |
| Yes | 170 (25.5) | ||||
| Chronic hepatic disease (N=397) | |||||
| No | 288 (28.9) | 0.000 | 2.255 (1.681–3.025) | 0.189 | - |
| Yes | 109 (47.8) | ||||
| Central nervous system disease (N=397) | |||||
| No | 167 (23.2) | 0.000 | 2.785 (2.177–3.562) | 0.983 | - |
| Yes | 230 (45.6) | ||||
| Cardiovascular disease (N=397) | |||||
| No | 229 (22.7) | 0.000 | 12.472 (8.7201–7.838) | 0.913 | - |
| Yes | 168 (78.5) | ||||
| COPD (N=397) | |||||
| No | 346 (30.1) | 0.000 | 4.938 (2.991–8.151) | 0.684 | - |
| Yes | 51 (68.0) | ||||
| Antiulcer drugs (N=397) | |||||
| No | 255 (24.0) | 0.000 | 23.711 (14.395–39.055) | 0.001 | 8.815 (2.474–31.402) |
| Yes | 142 (88.2) | ||||
| Antiepileptic drugs (N=397) | |||||
| No | 337 (29.2) | 0.000 | 14.564 (7.368–28.787) | 0.685 | - |
| Yes | 60 (85.7) | ||||
| Psychotropics (N=397) | |||||
| No | 190 (20.5) | 0.000 | 9.046 (6.734–12.153) | 0.000 | 5.639 (2.276–13.975) |
| Yes | 207 (69.9) | ||||
| COPD treatment (N=397) | |||||
| No | 342 (29.5) | 0.000 | 10.936 (5.783–20.679) | 0.132 | - |
| Yes | 55 (82.1) | ||||
| Cardiovascular (including antihypertensive) treatment (N=397) | |||||
| No | 210 (21.0) | 0.000 | 19.590 (13.293–28.871) | 0.010 | 10.276 (1.743–60.580) |
| Yes | 187 (83.9) | ||||
| Qualitative variable | Polypharmacy n (%) | p univar | Ajusted OR (CI 95%) | p multivar | Ajusted OR (CI 95%) |
|---|---|---|---|---|---|
| Dyslipidemia treatment (N=397) | |||||
| No | 238 (23.8) | 0.000 | 7.842 (5.677–10.834) | 0.611 | – |
| Yes | 159 (71.0) | ||||
| Oral antidiabetics (N=397) | |||||
| No | 336 (29.1) | 0.000 | 14.851 (7.519–29.332) | 0.427 | – |
| Yes | 61 (85.9) | ||||
| Presence of potential interaction (N=397) | |||||
| No | 95 (11.9) | 0.000 | 18.221 (13.502–24.589) | 0.000 | 6.974 (3.035–16.029) |
| Yes | 302 (71.1) | ||||
| Quantitative variable(U-Mann–Whitney) | Polifarmacy median (IQR) | p univar | Ajusted OR (CI 95%) | p multivar | Ajusted OR (CI 95%) |
|---|---|---|---|---|---|
| Ccomplexity index total score | 14 (11–18) | 0.000 | 9 (8.5–10) | 0.000 | 2.399 (2.041–3.311) |
| Number of comorbidities | 3 (2–4) | 0.000 | 2 (2–3) | 0.030 | 1.511 (1.014–2.193) |
| Number of comorbidity patterns | 1 (0–2) | 0.000 | 1 (1–1) | 0.279 | – |
| Number of polypharmacy patterns | 0 (0–1) | 0.000 | 0 (0–0) | 0.002 | 9.895 (2.346–41.738) |
COPD: Chronic Obstructive Pulmonary Disease.
This cross-sectional study indicates that polypharmacy is frequent in PLWH attending a hospital pharmacy service on an outpatient basis in Spain. So, we found that 32% of PLWH exhibited polypharmacy, a figure that is in the upper limit of the range reported in the literature and is similar to that reported in studies conducted in the United States 35.0%,8,11 32.2% Canada,9 30.8% Italy,10 and more recently, in a population-based study in a Spanish region 32.9%.23 Meanwhile, other countries such as the United Kingdom 21.0%,7 23.0% Australia,12 24.4% Switzerland6 and 23.7% Japan,24 have reported a lower prevalence of polypharmacy among PLWH (21–24%).
Previous studies conducted in our country were focused on an older population of PLWH and therefore reported higher figures of polypharmacy.13,25 Gimeno-Gracia et al., in a single Spanish center found that over 40% of PLWH were receiving 5 or more drugs with a daily defined dose>1.25 However, this study was conducted among 225 PLWH aged 50–64 years, while in our study, only 48% of the patients were 50 years or older.
The most frequent patterns of polypharmacy were cardiovascular and anxiety-depression. This is consistent with the fact that the most frequent comorbid conditions were central nervous disease disorders and hypertension/cardiovascular disease. Although the methods of reporting polypharmacy patterns or simply concomitant medications differ among studies, almost all studies, regardless of the setting, uniformly reported that the most frequent medications received by PLWH were cardiovascular drugs (e.g., statins, beta blockers, angiotensin-converting enzyme inhibitors) and drugs included in the anxiety-depression pattern (e.g., antidepressants, benzodiazepines and analgesics).6,7,11,12,24,26,27 Because we did not include a control group of non-HIV patients, it is not possible to evaluate whether this polypharmacy differs from that of the general population. However, Ware et al., in a study conducted in the US from 2004 to 2016, found that the use of antidepressants, lipid-lowering drugs and steroids was higher among PLWH while the use of antihypertensive drugs was almost identical in the two groups.6 This finding suggests that polypharmacy in PLWH could be due to aging. Regardless of whether it is related or not to HIV infection and its ART, the use of these medications increases the risk of drug-drug interactions,13,14,28,29 an important issue for managing these patients. Drugs perceived as safe, such as antiulcer drugs, are also frequent in PLWH. These drugs are associated with major problems such as osteoporosis or increased risk of Clostridium difficile infection or pneumonia, so they are candidates for deprescription.30
Treatment complexity has been scarcely investigated in PLWH.31 Our results suggest that both ART and other medications contributed almost equally to treatment complexity since the median MRCI score was 3 for both of them. This is in contrast with previous findings. Thus, Metz et al. analyzed data from the electronic records of US adult HIV-infected patients (2011–2012) and found that ART contributed approximately 25% to what they called the patient-level medication regimen complexity index, while other medications contributed approximately 66%.32 It is possible that these differences could be related to the index itself. The patient-level index compiles complexity scores for all patient medications, and the score is divided into equally weighted components (the ART regimen, other prescription medications, and over-the-counter medications), while we did not account for over-the-counter medications in our analysis. We have previously shown that the MRCI is an independent factor associated with treatment adherence suggesting that it is an important factor for identifying patients at risk of non-adherence.16 Although we did not analyze the relationship between medication complexity and adherence, consistent with previous reports, we found that adherence to prescribed medication was poor, with almost half of the patients being non-adherent to antiretroviral therapy and a similar proportion being non-adherent to other prescription medications. However, it should be taken into account that the criteria for adherence based on the SMAQ questionnaire are fairly stringent. In addition, this relationship between treatment complexity and treatment adherence could be modified by other factors, such as the presence of depression33 or cognitive impairment.34
This study have some limitations. First, this is a cross-sectional design, which, albeit adequate for evaluating the prevalence of polypharmacy, does not allow us to establish a causal relationship between the independent factors analyzed and the presence of polypharmacy. Another limitation could be that it is underestimated the frequency of polypharmacy because the patients could be receiving medication prescribed from private health insurance programs or clinics. On the other hand, our main strength is having carried out a national study, which undoubtedly allowed us to include a large number of patients.
In conclusion, our study shows that polypharmacy is frequent among Spanish PLWH and contributes to a relevant extent to treatment complexity. Due to the potential impact of polypharmacy on the occurrence of adverse outcomes, treatment adherence and possibly treatment success it is necessary to develop specific programs for addressing this issue and optimizing treatment of these patients. These programs will require an integrative approach, and therefore, we think the pharmacy department is an appropriate setting for its development and implementation.
Consent to participateInformed consent was obtained from all individual participants included in the study.
FundingThis work was supported by Merck Sharp & Dohme [“National multicenter cross-sectional observational study to determine the prevalence and complexity factors associated pharmacotherapy of polypharmacy in HIV+ patients in Spain” IISP 54910, 74.810 €].
Conflicts of interestRM-V has received a research grants from MSD for this study; JS-R has been advisory board member for MSD, Abbvie, and ViiV, and has received speaker honorarium from BMS, Gilead, and BD, and has received research grants from MSD; MG-G has been advisory board member for ViiV, and Abbvie, has received speaking honorarium from Ipsen, Jannsen, Gilead, and Roche, has received research grants from MSD, and has received travel grants from Roche, Lilly, Gilead, and ViiV; MAR-C has received research grants to institution from MSD, and advisory honorarium from ViiV; CA-G has no conflict of interest related with this manuscript.
We thank to Fernando Rico-Villademoros for his editorial assistance; this assistance has been funded by Spanish Society of Hospital Pharmacy.
Abad Lecha E, Antón Torres R, Arias Moya MA, Arrondo Velasco A, Arroyo Domingo E, Bernabéu Castella S, Bonilla Peñarrubia R, Campos Dávila E, Capilla Montes C, Cardona Peitx G, Casas Arrate J, Casás Martínez A, Cendon Otero MA, Cid Silva P, Climent Ballester S, Comas Sugrañes D, Company Bezares F, Cuellar Monreal MJ, Curiel García O, De la Llama Celis N, Devesa García C, Domingo Ruíz MA, Domínguez Recio A, Estefanell Tejero A, Fernández Espínola S, Fernández Lison LC, Fernández-Pacheco M, Floristan Imizcoz C, García Collado C, García Coronel M, García Salom P, García Simón MS, García Yubero C, Gil Candel M, Gil Mañez E, Gimeno Gracia M, Gómez de Segura Iriarte L, Gómez Germa P, González J, González Ponce CM, Hermegildo Caudevilla M, Henares López A, Huertas Fernández MJ, Iglesias Lambarri A, Illaro Uranga A, Jiménez Nácher I, Juan Aguilar M, Lázaro López A, Lázaro López E, Lebrero García A, Linares Alarcón A, López Aspiroz E, López-Gónzalez A, López Rodríguez I, López Sánchez P, Losa López L, Luque Pardo S, Marín Ventura L, Márquez Fernández E, Martin Conde MT, Martín Rizo L, Martínez E, Martínez Sesmero JM, Martínez Torrón A, Masip Torne M, Medina Comas R, Menéndez Naranjo L, Merino Martin V, Minguez Cabeza AC, Molina Cuadrado E, Monte Boquet E, Mora Atorrasagasti O, Moreno Villar A, Morillo Verdugo RA, Moya Carmona I, Murgadella i Sancho A, Navarro Aznárez H, Navarro Vilasaró M, Nazca Casariego GJ, Nieto Guindo M, Obaldia Alaña P, Ontañón Nasarre A, Ortega García P, Ortega Valín L, Parada MA, Pascual Barriga M, Pérez Campos M, Pérez Huertas P, Pérez Rodríguez N, Plaza Aniorte J, Prada Lobato JM, Prats Oliván P, Polache Vengud P, Puig Comas G, Retamero Delgado A, Ribes Artero H, Ríos Sánchez E, Rodríguez Gómez A, Rodríguez González C, Rodríguez Lucena FJ, Rodríguez Sagrado MA, Roldán Morales JC, Rosado María MC, Ruiz D, Ruiz González L, Sala Rodó M, Sánchez Garre MJ, Sánchez-Sánchez T, Sánchez Ulayar A, Sánchez-Rubio Ferrández J, Sandoval Fernández del Castillo S, Santana Martínez S, Santiago Pérez A, Serrano López de las Hazas JI, Soler Soler MM, Soriano Irigaray L, Soriano Martínez M, Tajes González YM, Tarazona Casany MV, Urbina Bengoa O, Valverde Merino MP, Velasco Costa J, Villa Rubio JA, Yurrebaso Eguilior N.