A 78-year-old male was admitted to our hospital with an extra-axial space-occupying lesion suggestive of posterior fossa meningioma, which was later confirmed by nuclear magnetic resonance. Surgery was performed to remove the meningioma and to insert an external ventricular drain to remove excess cerebrospinal fluid (CSF) due to developing secondary hydrocephalus, with subsequent admission to the intensive care unit (ICU).
On day 13 in the ICU, the device was changed after the first device malfunctioned due to the presence of a clot blocking the ventricular portion of the catheter. On day 26, the patient experienced an accelerated decline in neurological function with a blood-like fluid observed in the drain, which was sent for microbiological study. Empirical antibiotic therapy was initiated with meropenem and linezolid.
Biochemistry testing of the fluid suggested a bacterial infection due to the presence of pleocytosis (1320 cells/ml), with 90% polymorphonuclear lymphocytes, low glucose levels (0.2 g/l) and high protein (1.5 g/l) and lactic acid (1.1 g/dl) levels.
A gram stain showed abundant polymorphonuclear leukocytes and gram-negative rods of variable length with no specific morphology. In view of these findings, it was decided to perform a molecular study using multiplex PCR (FilmArray®, BCID panel, bioMérieux), based on the recommendations of Micó et al.,1 which was negative. Subsequently, a sample was cultured on blood agar, MacConkey agar and chocolate agar and incubated at 37 °C in aerobic conditions and 5% CO2, respectively.
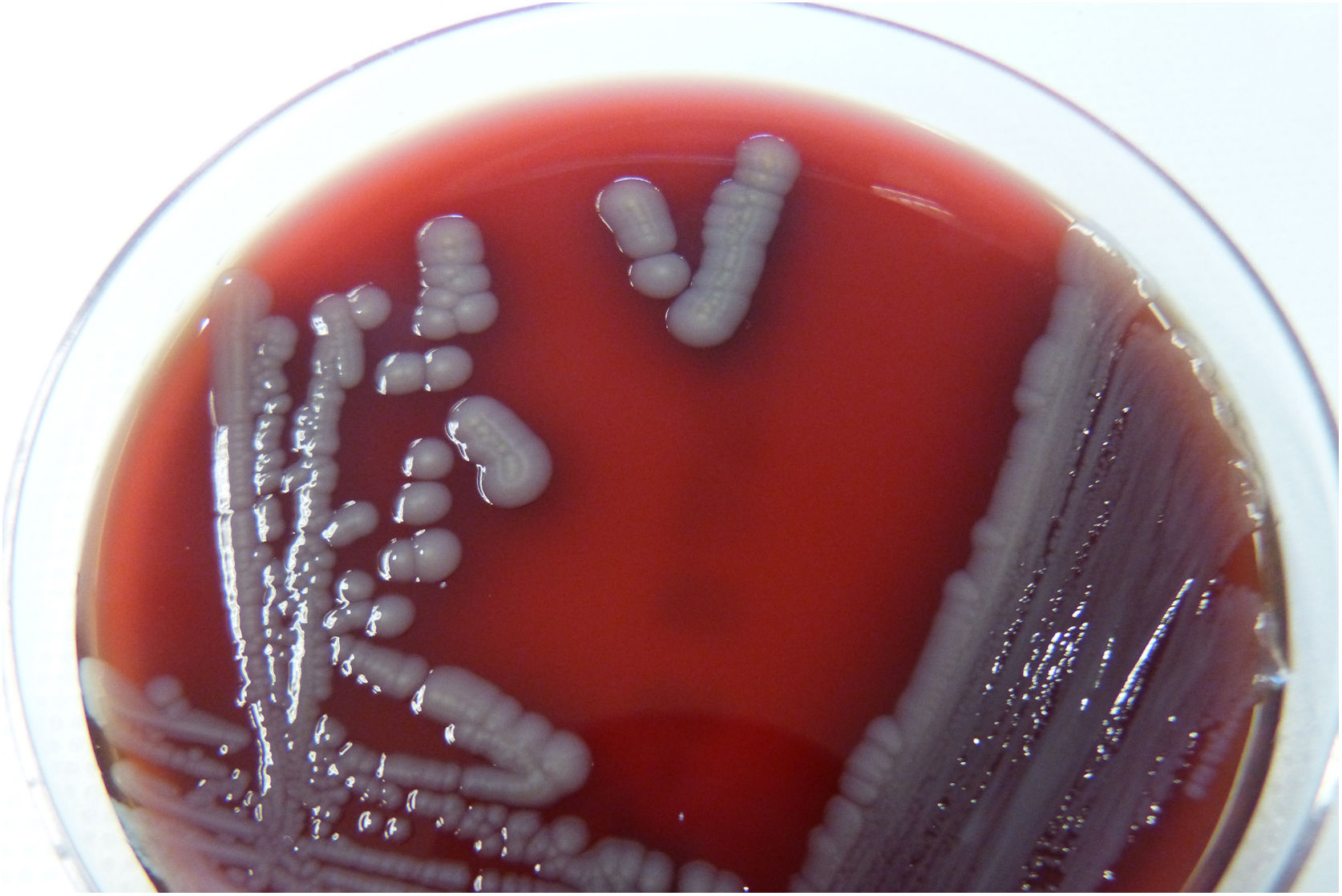
After 18 hours, growth in pure culture of non-pigmented mucoid colonies was observed on all three media (Fig. 1). The oxidase test was positive. The isolate was identified as Pseudomonas fluorescens/putida (99.9% probability) using a MicroScan Combo Panel, Type 71 (Beckman-Coulter, USA). It demonstrated sensitivity to standard doses of: meropenem, amikacin, tobramycin and colistin, and sensitivity with increased exposure to: piperacillin, ceftazidime, cefepime, ciprofloxacin and imipenem, based on EUCAST criteria.2 Duplicate mass spectrometry (Microflex LT, Bruker Daltonics, USA) identified the isolate as Pseudomonas monteilii, with values of 2.25 and 2.10 using matrix only and values of 2.34 and 2.19 with pre-treatment with formic acid.
The final diagnosis was meningoencephalitis caused by P. monteilii as a result of infection of the drainage device and treatment was changed to ceftazidime, resulting in a rapid clinical improvement, which was confirmed by negative CSF culture results at 72 h.
The genus Pseudomonas is divided into three phylogenetic lineages and at least 19 groups and sub-groups. One of the most relevant groups is the Pseudomonas putida group, which comprises up to 15 strains, including P. monteilii,3 which was first described in 1997.4 Phenotypically it easily fits into this group based on Pickett’s and Gilardi’s identification schemes,5,6 falling into the fluorescent group of non-fermenting gram-negative bacilli (GNB), together with Pseudomonas aeruginosa and Pseudomonas fluorescens. This phenotypic characterisation is still valid, despite the reclassification of Pseudomonas based on RNA/DNA homology studies and 16S rRNA gene sequencing-based characterisation.7 Also, per routine clinical laboratory practices, P. monteilii can be quickly and safely identified using mass spectrometry.8
P. monteilii is an environmental microorganism in healthcare settings and is often isolated from sink, tap and shower surfaces.9 In this context, it must also be considered as a potential pathogen and, as such, it has also been cultured from clinical specimens such as bronchial aspirates, urine, stool, bile and blood.4 Nevertheless, its role as a cause of central nervous system (CNS) infections has been rarely reported. In addition, evidence of infection is not clearly demonstrated in many of the cases described and this could suggest that P. monteilii may have low pathogenicity, act as a coloniser and only be a source of infection in critically ill or immunocompromised patients or those who have biomedical devices.10
ConclusionsBased on the above, the growth of P. monteilii on culture media was associated with a nosocomial infection of the external drain valve, either due to colonisation from the patient’s skin or handling of the device by healthcare staff, with this acting as a portal of entry for this microorganism into the ventricular fluid.
FundingGuillermo Martín Gutiérrez has a Juan Rodés clinical research contract (JR19/00039) from Instituto de Salud Carlos III, Ministry of Economy and Competitiveness (Ministerio de Economía y Competitividad), and also an Action B - Clinical Researchers (Acción B Clínicos Investigadores) contract (B-0006-2019) for promoting research activities within Clinical Management Units of the Andalucian Health Service (Servicio Andaluz de Salud) 2019, Department of Health and Families (Consejería de Salud y Familias).
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Toledo H, Martín-Gutiérrez G, Lepe JA. Meningitis nosocomial por Pseudomonas monteilii en paciente portador de catéter intraventricular. Enferm Infecc Microbiol Clin. 2022;40:92–93.