Staphylococcus caprae (S. caprae) is a gram-positive coagulase-negative, catalase-positive coccus, which was first described in 1958 as a skin and mammary glands coloniser in goats.1 It is considered a saprophytic flora that usually resides on the skin, nails and nasal mucosa.2 However, since the first case was described in 1983, its pathogenic role has been considered the cause of infections in different locations —peritonitis, meningitis, urinary tract infections, endocarditis, endophthalmitis, prosthetic joint infections, recurrent sepsis, bacteraemia, and osteomyelitis—. Risk factors include immunosuppressed states, obesity, traumatic or open fractures and especially contact with sheep or goats.3
As far as we can tell, the vast majority of cases are infections that settle on orthopaedic devices. The nosocomial origin of the infection, although difficult to prove, has been described within neonatal intensive care units, central line associated bacteraemia and after orthopaedic surgery.
Molecular techniques and matrix assisted laser desorption ionisation-time of flight mass spectrometry (MALDI-TOF MS) have led to an improvement in the identification of clinically relevant strains of S. caprae.4
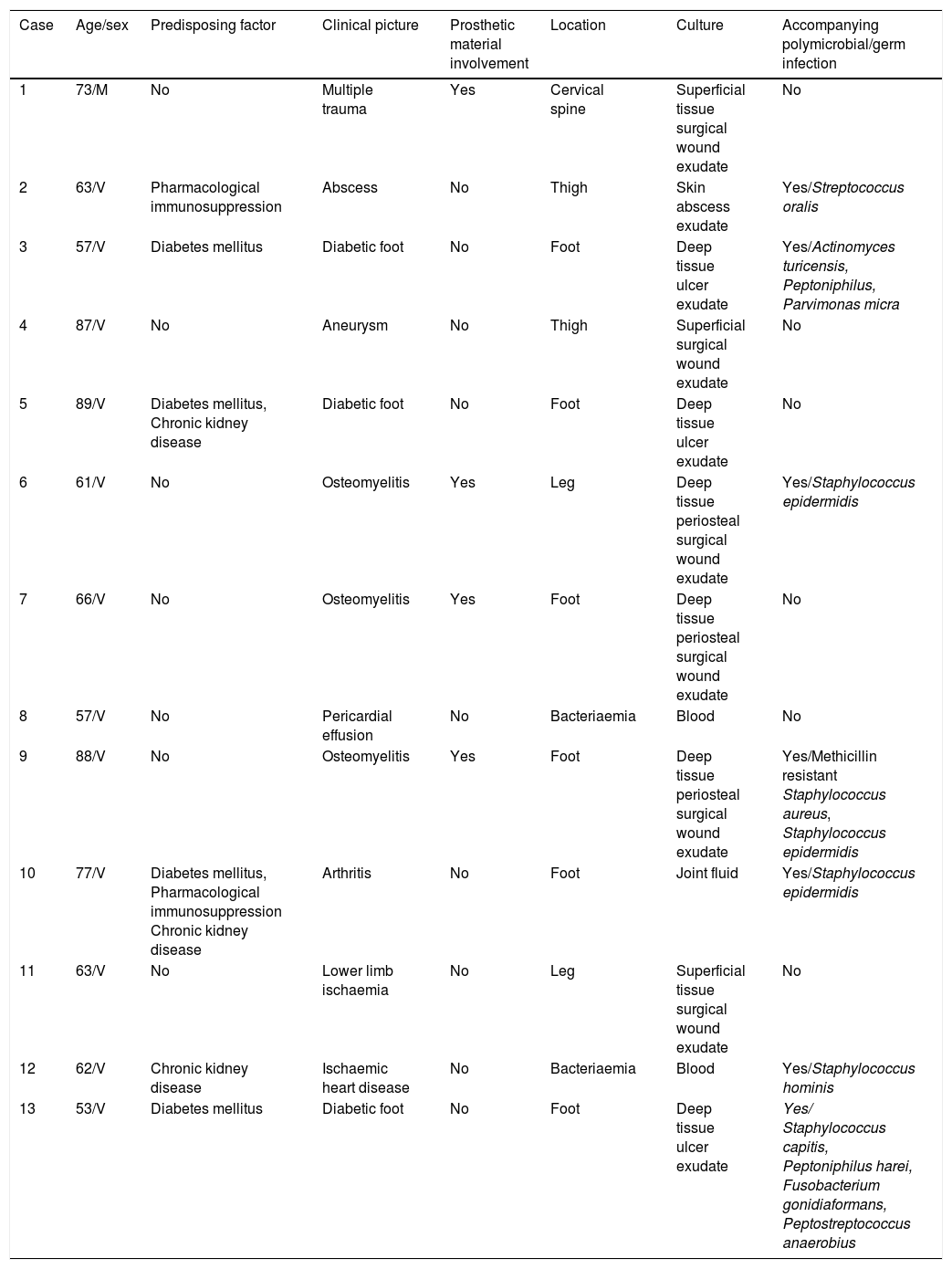
In the last 10 years we have had the opportunity to attend to 13 cases of infections due to S. caprae (Table 1). Their mean age was 69 years (SD 12.9) with a clear male predominance (91%).
Characteristics of the series.
| Case | Age/sex | Predisposing factor | Clinical picture | Prosthetic material involvement | Location | Culture | Accompanying polymicrobial/germ infection |
|---|---|---|---|---|---|---|---|
| 1 | 73/M | No | Multiple trauma | Yes | Cervical spine | Superficial tissue surgical wound exudate | No |
| 2 | 63/V | Pharmacological immunosuppression | Abscess | No | Thigh | Skin abscess exudate | Yes/Streptococcus oralis |
| 3 | 57/V | Diabetes mellitus | Diabetic foot | No | Foot | Deep tissue ulcer exudate | Yes/Actinomyces turicensis, Peptoniphilus, Parvimonas micra |
| 4 | 87/V | No | Aneurysm | No | Thigh | Superficial surgical wound exudate | No |
| 5 | 89/V | Diabetes mellitus, Chronic kidney disease | Diabetic foot | No | Foot | Deep tissue ulcer exudate | No |
| 6 | 61/V | No | Osteomyelitis | Yes | Leg | Deep tissue periosteal surgical wound exudate | Yes/Staphylococcus epidermidis |
| 7 | 66/V | No | Osteomyelitis | Yes | Foot | Deep tissue periosteal surgical wound exudate | No |
| 8 | 57/V | No | Pericardial effusion | No | Bacteriaemia | Blood | No |
| 9 | 88/V | No | Osteomyelitis | Yes | Foot | Deep tissue periosteal surgical wound exudate | Yes/Methicillin resistant Staphylococcus aureus, Staphylococcus epidermidis |
| 10 | 77/V | Diabetes mellitus, Pharmacological immunosuppression Chronic kidney disease | Arthritis | No | Foot | Joint fluid | Yes/Staphylococcus epidermidis |
| 11 | 63/V | No | Lower limb ischaemia | No | Leg | Superficial tissue surgical wound exudate | No |
| 12 | 62/V | Chronic kidney disease | Ischaemic heart disease | No | Bacteriaemia | Blood | Yes/Staphylococcus hominis |
| 13 | 53/V | Diabetes mellitus | Diabetic foot | No | Foot | Deep tissue ulcer exudate | Yes/ Staphylococcus capitis, Peptoniphilus harei, Fusobacterium gonidiaformans, Peptostreptococcus anaerobius |
The most common location was in the lower limbs (77%), followed by central line catheter associated bacteraemia (15%) and vertebral involvement (8%). Bone and/or joint involvement was observed in 54% with orthopaedic prosthetic material involvement of 31%, finally resolving in all cases.
At diagnosis, 46% had immunosuppressed states, and of all of those 67% had type 2 diabetes mellitus, 50% chronic kidney disease and 33% pharmacological immunosuppression secondary to corticosteroid consumption. The Charlson Index was greater than 5 points in 61% of cases.
In 54% of the cases the infection turned out to be polymicrobial with a predominance of association with cocci and gram-positive bacilli, while in the remaining 46% it was monomicrobial. Regarding antibiotic sensitivity only 8% showed resistance to fluoroquinolones and 23% to penicillins. Patients were mainly treated with beta-lactams (46%) and fluoroquinolones(31%); to a lesser extent, the use of glycopeptides (8%) and oxazolidinones (15%) was observed. Antibiotics were maintained for a mean of 29 days, excluding the case of joint involvement due to transfer of the patient to the reference centre. In 31% of the patients sequential therapy was possible, thus making treatment on an outpatient basis possible. In 92% of cases it presented as a nosocomial infection. No case of death was documented during the infectious process or 30 days after medical discharge.
S. caprae has been described as a potential pathogen with special voracity for orthopaedic devices in immunocompromised hosts.4 However, in our series we did not observe differences regarding the immunosuppression status, and in just 31% of cases did the infection involve orthopaedic prosthetic material. An association was observed in patients with greater comorbidity, but despite this, its mortality was low, which suggests low aggressiveness as a pathogen. Like other microorganisms in the coagulase-negative group,5S. caprae is mainly related to bone, joint and bacteraemia involvement. In summary, S. caprae is a primarily hospital-acquired microorganism whose pathogenic potential is currently underestimated, so knowledge and early detection would help to control this emerging pathogen.
Please cite this article as: Rodríguez Fernández L, Martín Guerra JM, Dueñas Gutiérrez CJ. Papel del Staphylococcus caprae en la infección nosocomial. Enferm Infecc Microbiol Clin. 2020;38:455–456.