This is the case of a 55-year-old patient with type 2 diabetes mellitus and microangiopathy treated with metformin (850mg twice daily). He also had open-angle glaucoma, a diabetic retinopathy associated with macular oedema for which he had received 14 intravitreal injections in his right eye (RE) and eight injections in his left eye (LE) with aflibercept (40mg/ml), as well as retinal detachment in his LE that required pars plana vitrectomy (23g and endolaser for small paravascular tears). In addition, he reported frequent use of daily contact lenses. The patient suffered from progressive worsening of visual acuity in his RE with conjunctival hyperaemia and photophobia. Examination of his RE revealed a cloudy cornea, as well as two lateral corneal ulcers (3×2mm) and another in the medial region in a crescent shape. Corneal scraping samples were taken for microbiological study.
The corneal scraping sample was inoculated on trypticase soy agar with 5% sheep blood (Becton Dickinson, Franklin Lakes, NJ, USA), Sabouraud agar with chloramphenicol (BD™) and chocolate agar, in addition to specific culture for the detection of Acanthamoeba spp. For the culture of Acanthamoeba spp., one to two colonies of Escherichia coli or Enterobacter aerogenes were emulsified in Page’s solution until a homogeneous turbidity was achieved, and 0.2ml of this solution was then inoculated in a Petri dish. Subsequently, the sample was inoculated in this medium and visualised every 24–48h, with growth observed after four days Fig. 1b, example of growth in Page’s medium). A positive PCR for Acanthamoeba spp. confirmed the result. Treatment was started alternately with propamidine (0.1%/10ml every two hours) and chlorhexidine (0.02%/10ml every two hours) eye drops.
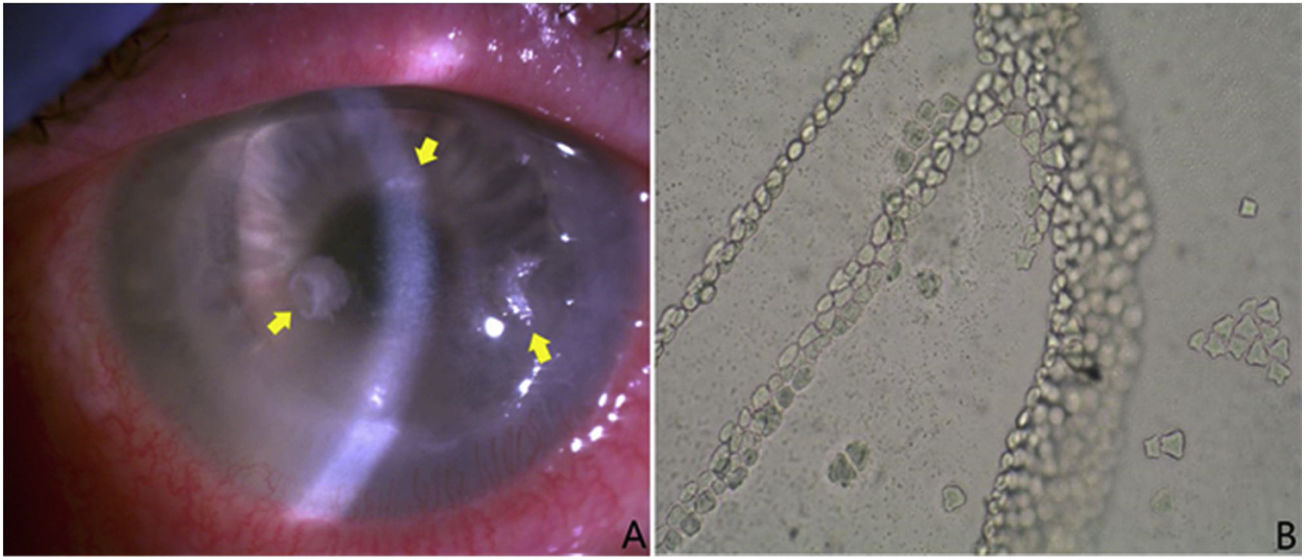
(A) Three thinned lesions with sublesional endothelial halo and with a crystalline appearance (yellow arrows). Samples were taken from them and were sent to the microbiology laboratory for culture and anatomical pathology for study. These lesions were colonised by S. oralis. (B) Typical growth of Acanthamoeba spp. cysts in Page’s medium covered with Escherichia coli. Presence of laminae contiguous with cysts. Without staining and with magnification ×100.
The clinical evolution was initially good, but the patient subsequently started to experience progressive worsening, with persistent corneal ulcer and annular infiltrate. In addition, three thinned lesions with a sublesional endothelial halo and with a crystalline appearance (Fig. 1a) appeared and the decision was taken to perform another corneal scraping for culture. The scrape sample was re-cultured in the same culture media as above. In this case, growth of Streptococcus oralis was observed, susceptible to penicillin, vancomycin and tetracyclines, whereupon treatment with eye drops with moxifloxacin was initiated (5mg/ml every six hours), which was later replaced by eye drops reinforced with vancomycin and penicillin eye drops (50mg/ml and 330,000U/ml, respectively, every two hours alternately). In the anatomical pathology, colonies of compacted bacterial-like microorganisms were observed in the most superficial third, as well as cysts consistent with infestation by Acanthamoeba spp.
In the view of the lack of improvement, a penetrating keratoplasty was performed after a month and a half of treatment, with a corneal suture required one week later due to positive Seidel (communication between the anterior and exterior chamber). The surgically obtained corneal sample was processed by microbiological cultures, as well as PCR for Acanthamoeba spp. and 16S rDNA sequencing, being negative for all tests. Following the penetrating keratoplasty, the patient presented a progressive clinical improvement and close follow-up by the Ophthalmology department showed clearing of the cornea.
DiscussionInfectious crystalline keratopathy is a form of indolent infectious keratitis characterised by characteristic needle-shaped opacities associated with minimal or no inflammatory reaction. Some authors call it “non-inflammatory intrastromal bacterial colonisation” and avoid the term “infection” due to the absence of inflammation. It was first described by Gorovoy et al.1 when gram-positive cocci were discovered colonising the cornea of a patient after penetrating keratoplasty. Apparently, the microorganisms enter the corneal stroma through an epithelial defect that can be exploited by virulent or opportunistic pathogens which proliferate. When the usual immune response is attenuated by localised immunosuppression, it allows microorganisms to be eventually surrounded, sometimes by a biofilm2.
Characteristically, the most common causal agents of this syndrome are Streptococcus alpha-haemolytic cells of the viridans group, especially, Streptococcus mitis2–4. This association between Streptococcus spp. and crystalline keratopathy could be related to the possible abundant production of a mucopolysaccharide biofilm, although more studies are needed to confirm this association4. Other pathogens that can be the cause of this pathology are other gram-positive cocci (coagulase-negative Staphylococcus, Enterococcus faecalis…), and to a lesser extent, gram-negative bacilli and fungi such as Candida albicans, among others2,3,5,6.
In most cases, the necessary epithelial defect appears after surgery associated with the use of topical corticosteroids (frequent after corneal surgeries)1. The use of a sterile field or the correct cleaning and sterilisation of the instruments, the detection of risk factors (such as blepharitis or tear duct obstruction) and the use of povidone-iodine could reduce the colonisation and participation of opportunistic pathogens such as S. oralis. The use of local anaesthetics, as well as previous keratitis due to Achantamoeba spp. (usually after contact lens wear), is also associated with crystalline keratopathy2–4. Another hypothesis indicates that microorganisms could establish an endosymbiotic relationship with Acanthamoeba spp. and when the amoebas begin to die after the use of antiseptics they are released from their interior and attach to the cornea7.
A corneal scraping should be carried out to identify the causative microorganism, although sometimes the depth of the lesions will not be reached and a corneal biopsy may be necessary to confirm the diagnosis8. Another diagnostic option is to perform a fine needle aspiration and PCR, which would also avoid the use of invasive techniques.
The first therapeutic option is topical broad-spectrum antibiotics, which must be reinforced to try to cross the biofilms formed by some microorganisms2,9. When there is no improvement, the intrastromal route should be chosen10. The use of laser (excimer laser or Nd:YAG) as an adjuvant therapy to topical treatment can be useful to prevent the formation of biofilm6. In the event of disease case progression, surgical excision of the infiltrated tissue appears to be necessary. Anterior lamellar dissection is performed for more superficial lesions, while penetrating keratoplasty is reserved for deeper lesions (the entire cornea is replaced as opposed to lamellar or selective keratoplasty, if only the affected layers are replaced)9.
Crystalline keratopathy requires rapid diagnostic and therapeutic action, and will sometimes require other complementary actions such as the use of laser or surgery to eliminate the settled bacteria completely. Limiting the use of corticosteroids and/or local anaesthetics, together with close clinical follow-up after this type of surgery, is deemed essential.
FundingThis text has not received funding.
AuthorsDomingo Fernández Vecilla: drafted the scientific text, reviewed the literature.
Silvia López-Plandolit Antolin: helped draft the case report, reviewed the literature, and provided the images.
Miren Josebe Unzaga Barañano: helped with the conception of the case, reviewed it and helped to modify it.
José Luis Díaz de Tuesta del Arco: reviewed the case, helped to modify it, and reviewed the literature.
Conflicts of interestThe authors declare that they have no conflicts of interest.