Today there are multiple types of flu vaccines. The emergence of nucleic acid technology used in vaccines against SARS-CoV-2 suggests its future application against this infection. Against influenza, two types of vaccines have been developed based on messenger RNA (mRNA): conventional or non-replicative and self-amplifying or replicative (auRNA), both included in lipid nanoparticles. Animal studies carried out with the former have shown their strong capacity to induce Th-1 antibodies and cellular immunity against influenza haemagglutinin (HA) with few side effects. Human trials have shown 87% seroconversion and 100% seroprotection. The auRNA vaccines have obtained similar results in animals but at a concentration 64 times lower than the conventional one. Vaccines based on mRNA platforms meet the WHO requirements for next generation influenza vaccines.
En la actualidad existen múltiples tipos de vacunas frente a la gripe. La irrupción de la tecnología de ácidos nucleicos utilizada en las vacunas frente al SARS-CoV-2 hace pensar en su aplicación futura frente a esta infección. Frente a la gripe se han desarrollado dos tipos de vacunas basadas en el ARN mensajero (ARNm): las convencionales o no replicativas y las autoamplificables o replicativas (auARNm), ambas incluidas en nanopartículas lipídicas. Los estudios en animales realizados con las primeras han mostrado su intensa capacidad para inducir anticuerpos e inmunidad celular Th-1 frente a la hemaglutinina (HA) gripal con escasos efectos secundarios. Los ensayos en humanos han mostrado una seroconversión del 87% y una seroprotección del 100%. Las vacunas auARNm han obtenido resultados en animales semejantes pero a una concentración 64 veces inferior a la convencional. Las vacunas basadas en las plataformas de ARNm cumplen los requisitos establecidos por la OMS para vacunas de gripe de la generación siguiente.
Influenza is one of the main viral acute respiratory infections that periodically affects the human population. Despite the fact that it is generally considered a mild disease, when it affects populations that are fragile or vulnerable due to underlying diseases it can present significant morbidity and mortality. Only some moderately effective antiviral drugs are available against influenza, whereby vaccination is the main and virtually the only way to protect the population from it. For this reason, different types and varieties of vaccines with variable efficacies have been developed1. The emergence of nucleic acid (RNA and DNA) technology used in vaccines against SARS-CoV-2 leads us to consider the possibility of it being applied against influenza in the future2,3.
Messenger RNA vaccinesTwo types of messenger RNA (mRNA) molecules with very different biological properties have been developed for use as vaccines: conventional or non-replicating and self-amplifying or replicating.
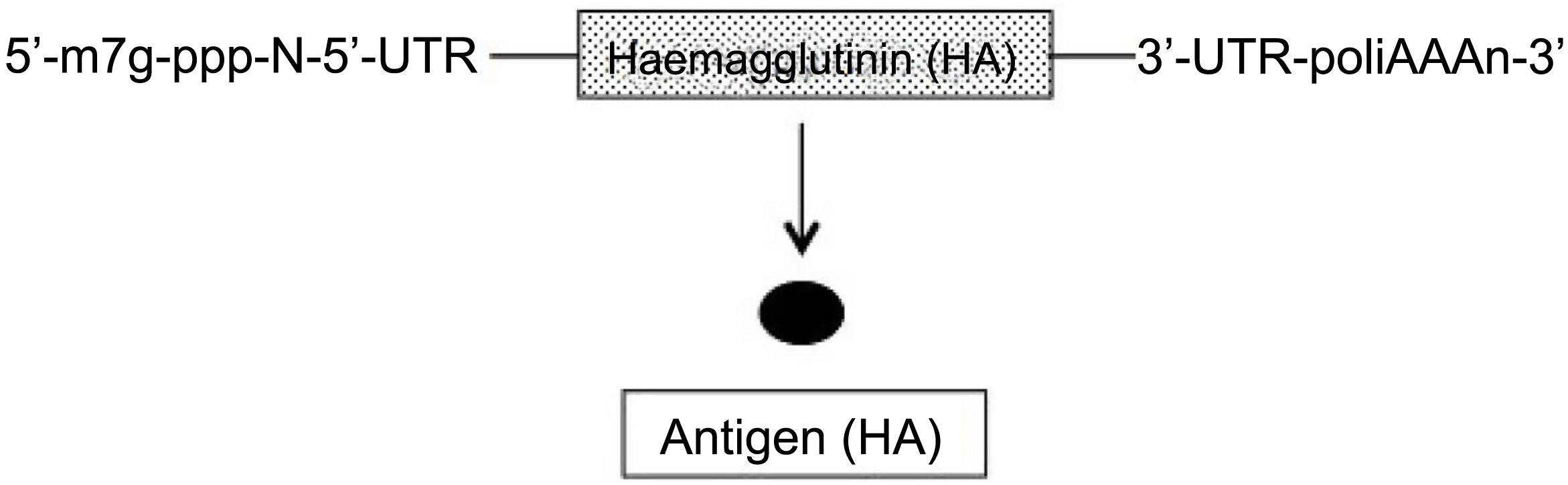
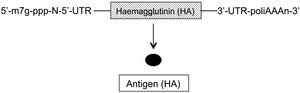
In conventional and non-replicating mRNA, similar to cell RNA, the genetic sequence of the protein (antigen) to be expressed is flanked on each of its 5′ and 3′ terminals by an untranslated region (UTR) sequence. Furthermore, like all mRNAs, at the 5′ terminal it has the so-called cap sequence (m7G-ppp-N-5′) and at the 3′ terminal a variable-length adenine sequence (polyA) (Fig. 1)2–4. Two types of these vaccines have been developed, those with unmodified (natural) mRNA and those with modified mRNA; in the latter, the uridine nucleoside has been replaced by 1-methyl-pseudouridine, which stabilises the molecule5. The advantages of mRNA are that it is a simple and small molecule (about 2–3 kb) and, since it encodes a single protein, the immune response is very specific. However, the expression of this mRNA (antigen production) is limited and by nature transient, requiring the administration of high doses to obtain good vaccine efficacy2–4.
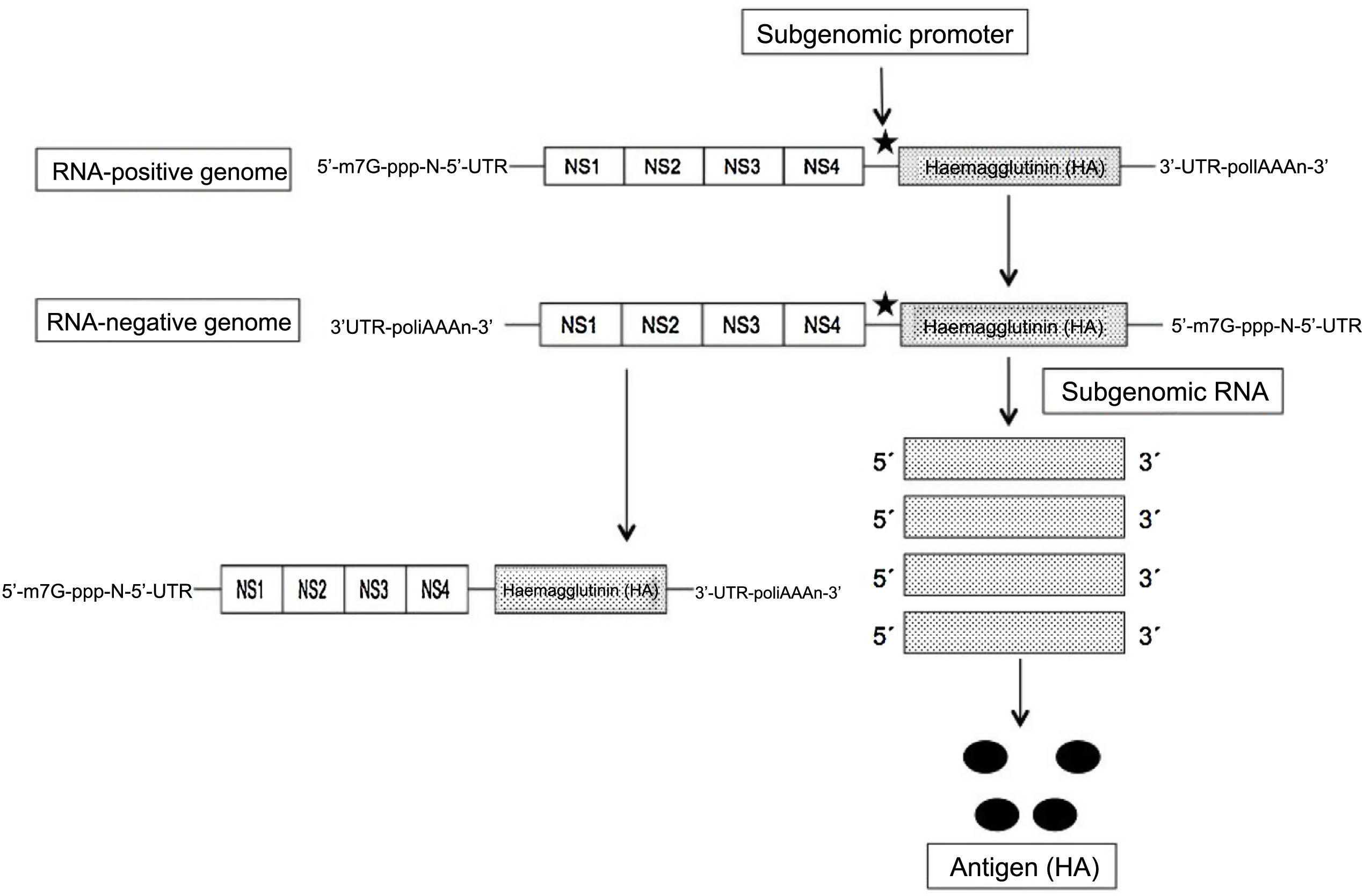
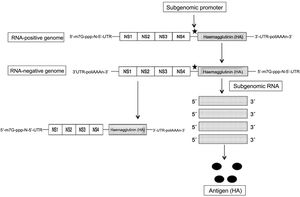
The self-amplifying and replicating mRNA (SAM RNA) is based on the use of the genome of another RNA-positive virus, such as an alphavirus or flavivirus, as a vector, and leveraging the set of genes that encode non-structural (NS) and essential proteins, including the RNA-dependent RNA polymerase (NS1), and eliminating the rest to replace it with the genetic sequence of the protein/antigen to be expressed. This yields a molecule of about 9 kb in length, called a “replicon”. After the initial replication of the virus in the cytoplasm, the subgenomic fragments that encode the desired antigen are expressed (thanks to the insertion of a promoter) (Fig. 2). In this way, and thanks to the self-replicating process of a single SAM RNA molecule, large amounts of antigen are obtained. Despite the use of genes from other viruses, they are not capable of forming viable particles and there is no risk of infection by them2–4. With this SAM RNA, lower concentrations are needed, and therefore also lower amounts of lipid nanoparticles that include them, inducing adverse effects that are also lower compared to conventional mRNA6. In this way, the SAM RNA vaccines only require 0.1–1.0 μg to obtain total protection, compared to 10 μg of the conventional one in mice immunised with the influenza haemagglutinin (HA)7. In addition, the RNA replicons formed in the amplification process present recognition patterns on the cell surface that increase immune response. Although both types of mRNA induce humoral and cellular immunity, the SAM RNA determines higher levels of expression and complex immune response8.
The fragility and rapid physiological degradation of mRNA molecules has made it necessary to protect them to be administered in mammals. The best solution to this problem has been to include them in a complex lipid structure of about 80 nm, similar to the influenza virus, forming what are called lipid nanoparticles (LNP)9,10.
mRNA influenza vaccinesmRNA influenza vaccines provide a series of advantages over other types of vaccines, such as: (a) a very favourable safety profile (RNA is a non-infectious molecule, it cannot be integrated into the cellular genome and is rapidly degraded by cytoplasmic RNAses); (b) a highly-controllable antigen production process with high antigen identification, since it is produced in a similar way to the viral replication process in natural infection by the human cell itself; (c) fast and scalable production, requiring little time for initial production or subsequent re-actualization; and (d) it does not require the use of embryonated eggs or cell cultures that could alter the antigenicity of the final protein6,11.
The first study on the efficacy of a conventional mRNA against influenza was carried out in 1993, showing how the administration of a liposomal vaccine of this type that encoded the influenza nucleoprotein (NP) induced a cytotoxic T-cell response in mice12. Subsequently, other authors demonstrated that administration to mice, ferrets and pigs of different mRNAs encoding HA, neuraminidase (NA) and NP, also induced a potent humoral response with a single dose11. In these studies, the mRNA-HA vaccines had been shown to be immunogenic in mice and to protect them from subsequent infection. To confirm this, Petsch et al.13 inoculated two doses of 80 μg of this vaccine into mice of all ages, observing lifelong protection. Pigs inoculated with a 250-μg dose of HA, NA, and NP vaccines also obtained and maintained protective titres similar to those induced by the equivalent inactivated vaccine.
Studies in non-human primates demonstrated that the intramuscular administration of a single 10-μg dose of an mRNA-LNP encoding the HA of the influenza A/Netherlands/602/2009 (H1N1) virus induced protective titres of inhibitory neutralising antibodies (NAbs) in a range >1:4014. The administration of a second dose determined the mean increase in titre above 1:160 in all the animals and for a minimum of one year. In addition, they observed how the intramuscular administration of two doses (four weeks apart) of the vaccine containing the HA of the influenza virus A/HongKong/4801/2014 (H3N2) induced an intense type T and B cell response higher than inactivated vaccines with MF59 adjuvant14.
Pardi et al.15 reported that the administration of a single 3-μg dose of an mRNA-LNP-HA vaccine with the A/California/07/2009 (H1N1) strain to mice and ferrets determined NAbs titres >1:120 four weeks after immunisation. A second dose increased these titres to values of 1,280–20,480, depending on the dose and route of administration (intradermal or intramuscular). The vaccine did not generate NAbs antibodies against the A/Puerto Rico/8/1934 (H1N1) strain, but it did protect the animals after infection, suggesting the induction of immune responses against the HA stalk (subsequently confirmed) and not against the immunodominant globular head domain. The inoculation of mice with a 50-μg dose of a tetravalent mRNA-LNP vaccine containing the genes for the HA, NA, NP and Matrix antigens demonstrated a robust immune response against the pandemic strain A (H1N1) about 500-fold that of a conventional vaccine. In addition, the antibody titres against each of the antigens were the same as those obtained with the separate inoculation. This demonstrated the possibility of developing a multiantigenic influenza vaccine with a broad protective spectrum16.
Bahl et al.17 observed that a single administration of 10 μg of a modified mRNA-LNP encoding the HA of the influenza virus A/Jiangxi-Donghu/346/2013 (H10N8) or A/Anhui/1/2003 (H7N9) in mice induced a humoral immune response that persisted beyond a year. In addition, a single dose of 0.4 μg of the virus (H7N9) was found to protect mice from subsequent infection with the same virus and a 10-μg dose dramatically reduced the presence of the virus in ferret lung parenchyma. They also observed how the administration of two doses (intramuscular or intradermal) of an mRNA-LNP-H10 vaccine in macaques induced protective inhibitor titres and specific CD4+ T cells. The vaccine induced a rapid and local infiltration of neutrophils, monocytes and dendritic cells at the point of inoculation and in local lymph nodes and persistent humoral and cellular immunity18,19.
On the basis of these results, Bahl et al.17 began a phase I clinical trial in a small group of volunteers (23 vaccinated and eight placebos) who were given two intramuscular doses of 100 μg of mRNA-LNP-H10. The seroconversion percentage was 87% (defined as the change from absence to presence of NAbs antibodies) and 87% (defined as a fourfold increase in titre). The percentage of seroprotection observed at 43 days was 100% (defined as the percentage of people who reached a NAbs antibody titre >40) and 87% in microneutralisation titres. Most of the adverse effects were mild and associated with the jab. These results showed that this new type of vaccine obtained protective efficacy results very similar to those of other conventional influenza vaccines17.
The safety and immunogenicity of an mRNA-LNP vaccine against the avian influenza viruses H10N8 and H7N9 was evaluated in a phase I clinical trial in humans. For H10N8, an intramuscular dose of 100 μg induced titres >1:40 in 100% of the participants, and for H7N9, intramuscular doses of 10, 25, and 50 μg induced titres >1:40 in 36%, 96.3%, and 89.7% respectively. Seroconversion rates for H10N8 were 78.3% and 96.3% against H7N9. This study confirms the immunogenicity of mRNA-LNP vaccines in humans and the scant adverse effects induced by them20.
The first studies on SAM RNA vaccines showed that the administration of 10 μg of the HA of the influenza A/Puerto Rico/8/34 (H1N1) virus induced a humoral response in mice that provided partial protection against subsequent influenza infection21. Hekele et al.6 developed a SAM RNA-LNP vaccine against the A/California/07/2009 (H1N1) and A/Shanghai/2/2013 (H7N9) viruses. Two doses of 0.1–1.0 μg induced levels of NAbs-type protective antibodies in mice similar to conventional vaccines. Magini et al.22 tested a SAM RNA-LNP vaccine encoding the NP and the M1 protein (internal matrix protein that envelops the virus above the nucleocapsid) from the A/Puerto Rico/8/34 (H1N1) strain. The intramuscular administration of 0.1–0.2 μg of the vaccine with the NP or M1 or the combined NP + M1 determined an intense response of specific T cells (CD4 Th1), associated with a certain subsequent protection. It also induced a robust expansion of cellular immunological memory and effector memory, necessary for long-term protection. The study by Brazzoli et al.8 shows how the administration of this type of vaccine against the HA of the A/California/07/2009 (H1N1) strain in mice and ferrets determined cross-protection against the homologous and heterologous strain (A/Puerto Rico/8/34) due to the induction of multifunctional CD8-T cells.
Vogel et al.23 compared the immune response and protective efficacy of a SAM RNA vaccine and a conventional mRNA vaccine (both of the LNP type) against influenza in mice. The animals were immunised with increasing doses of the HA of the A/Puerto Rico/8/34 (H1N1) strain. Both vaccine types induced protection against infection by the homologous strain. However, the SAM RNA vaccine was found to achieve the same level of immunological protection with a dose 64 times lower than the conventional one (1.25 μg versus 80 μg). In addition, immunisation with a trivalent SAM RNA vaccine containing the HAs of the A/California/07/2009 (H1N1), A/Hong Kong/1/68/ (H3N2) and B/Massachusetts/2/2012 strains protected animals against homologous viruses. For the first time, the efficacy and usefulness of SAM RNA-LNP vaccines formulated with more than one antigen component of two different types of influenza were demonstrated.
Despite the preliminary results obtained in the experimental models, no clinical trials have been initiated with the SAM RNA-type vaccine, whereby immune response and protective capacity in humans are unknown.
The requirements document for new or future influenza vaccines prepared by the WHO24 contains the following specifications: (a) prevention of severe laboratory-confirmed influenza A and B; (b) safety and efficacy studies in different age groups; (c) low levels of reactogenicity; (d) absence of interference with other vaccines; (e) duration of immunity between 1 and 5 years; (f) use of antigens capable of stimulating the cellular immune system; and (g) establishment of a protection correlate that makes it possible to evaluate the efficacy of the new vaccines.
mRNA vaccines meet most of these requirements, having demonstrated the capacity to protect animals from different influenza strains, although these data need to be transferred to humans. They are safe and have scant adverse effects, they can be re-actualized based on the antigen variation of influenza viruses and present an immune durability of at least one year. In addition, for production purposes, the virus does not have to be grown in cell cultures, only its identification, optimisation and the expression of the mRNA encoding the chosen antigen25. For all these reasons, it is very possible that following the success of mRNA vaccines against SARS-CoV-2, the new generation of vaccines against influenza will be based on this same nucleic acid molecular engineering model.
Conflicts of interestThe author has no conflicts of interest to declare.