Influenza poses a significant burden in terms of morbidity and mortality, with vaccination being one of the most effective measures for its prevention. Therefore, the aim of this study is to determine the effectiveness of the influenza vaccine in preventing cases of severe influenza in patients admitted to a tertiary hospital during the 2022/23 season.
MethodsCase-control study. All hospitalised patients with a positive result in an RT-PCR for influenza were included. Those who met the criteria for a severe case (pneumonia, sepsis, multi-organ failure, admission to ICU or exitus) were considered cases. Those who did not meet these criteria were considered controls. Vaccine effectiveness (VE) to prevent severe cases and its 95% confidence interval were calculated.
ResultsA total of 403 patients were admitted with confirmed influenza. Of these, 98 (24.3%) developed severe influenza. Of the total, 50.6% were men and 47.1% were over 65 years of age. VE adjusted for influenza type, age and certain comorbidities was 40.6% (−21.9 to 71.1). In a segmented analysis, influenza vaccine was effective in preventing severe cases in all categories. It was particularly relevant in the 65+ age group (VEa = 60.9%; −2.0 to 85.0) and in patients with influenza A (VEa = 56.7%; 1.5–80.9).
ConclusionInfluenza vaccination markedly reduced the occurrence of severe cases of influenza in hospitalised patients, therefore, it remains the main strategy to reduce morbidity and mortality and associated costs.
La gripe supone una importante carga en términos de morbimortalidad, siendo la vacunación una de las medidas más efectivas para su prevención. Por lo que el objetivo de este estudio es conocer la efectividad de la vacuna antigripal para prevenir casos de gripe grave en los pacientes ingresados en un hospital de tercer nivel durante la temporada 2022/23.
MetodologíaEstudio de casos y controles. Se incluyeron todos los pacientes hospitalizados con resultado positivo en una RT-PCR de Gripe. Se consideró caso a aquellos que cumplieron criterio de caso grave (neumonía, sepsis, fallo multiorgánico, ingreso en UCI o exitus). Quienes no los cumplían se consideraron controles. Se calculó la efectividad vacunal (EV) para prevenir casos graves y su intervalo de confianza al 95%.
ResultadosUn total de 403 pacientes ingresaron con gripe confirmada. 98 (24,3%) de ellos desarrollaron gripe grave. Del total, el 50,6% fueron hombres y el 47,1% fueron mayores de 65 años. La EV ajustada por tipo de gripe, edad y ciertas comorbilidades fue del 40,6% (−21,9 to 71,1). En un análisis segmentado, la vacuna de la gripe resultó efectiva para la prevención de casos graves en todas las categorías. Resultó especialmente relevante en el grupo de 65 años o más (EVa = 60,9%; −2,0 to 85,0) y en los pacientes con gripe A (EVa = 56,7%; 1,5–80,9).
ConclusionesLa vacunación antigripal redujo notablemente la aparición de casos graves de gripe en los pacientes hospitalizados, por tanto, sigue siendo la principal estrategia para reducir la morbimortalidad y los costes asociados.
Seasonal influenza represents a significant burden in terms of morbidity and mortality and health costs,1,2 causing up to 650,000 deaths each year worldwide.3 Although vaccine effectiveness (VE) against influenza has not reached very high levels in the seasons prior to the 2022/23 season,4 vaccination continues to be the most useful prevention strategy for reducing the impact of this disease5 and continues to be recommended particularly in patients aged over 65, patients with comorbidities and pregnant women.6
In the 2019/20 season in Spain, a very positive impact from vaccination was estimated in terms of prevention of serious cases and the reduction in influenza-related morbidity and mortality. It was calculated that the vaccine prevented 26% of hospital admissions, 40% of admissions to intensive care units (ICU) and 37% of deaths attributable to influenza in people aged over 65.7
As a consequence of the antigenic variability that the influenza virus undergoes each year, the VE changes each year.8 This can be seen by comparing the VE results from different seasons. The multicentre European I-MOVE study showed how VE varied slightly when the results of the 2019/2020 season9 were compared with those from 2020/2021.10 As a result, the composition of the vaccine also changes every year. With the data provided by worldwide influenza surveillance in the months prior to the start of the season, the composition is determined in each season so that there is the greatest possible similarity between the circulating strains and those included in the annual vaccine.11 The VE will therefore be higher or lower depending on how similar they are.
In the 2022/2023 season,12 the first estimates show a greater similarity with previous years, exceeding 50% VE in an unusually early season, in which influenza A (H3N2) predominated.
It is therefore important to know the impact of the vaccine in preventing infections each year and, at the same time, its ability to reduce severity in people who do develop disease. Despite the limited number of studies on the subject, there is increasing evidence of the vaccine preventing severe cases of flu and the ensuing consequences.13,14
Additionally, the COVID-19 pandemic has increased the number of admissions deriving from respiratory viruses, and has led to the implementation of the surveillance system for these, as well as preventive measures.15 In Spain, an acute respiratory infection surveillance system (Sistema de Vigilancia de Infección Respiratoria Aguda [SiVIRA]) was set up16,17 which has replaced the traditional flu surveillance system. The old system worked actively in the search for flu cases from week 40 of one year to week 20 of the next, with their corresponding inter-season periods (weeks 21–39). The new system monitors continuously throughout the year for influenza, COVID-19 and other respiratory viruses.
This study aims to demonstrate the benefits of vaccination, especially in the most vulnerable groups, even when vaccination does not prevent infection. This could help achieve better vaccine coverage in the high-risk population and so reduce the severity of cases, even in those who already have the infection and have been admitted to hospital.
To that end, the aim of this study is to determine the effectiveness of the flu vaccine in preventing severe cases in hospitalised patients during the 2022/23 season.
MethodsWe carried out an observational case-control study in a tertiary hospital. The study period covered from epidemiological week 40 of 2022 to week 20 of 2023. All patients over six months of age admitted to the hospital who had laboratory-confirmed influenza were included; those under six months of age were excluded as vaccination was not indicated for these patients. Those who met the criteria for severe influenza were considered as a case. Severe flu was defined as a case with laboratory-confirmed influenza who had at least one of the following during admission: ICU admission; pneumonia; septic shock; multiple organ failure; or death. To consider whether a patient had any of these clinical characteristics (pneumonia, septic shock or multiple organ failure), we checked their electronic medical records when collecting the data for each patient and, depending on the outcomes entered by the clinician, the patient was included as a case or as a control. Patients with laboratory-confirmed influenza who did not meet any of these severity criteria were considered as controls.
The collection of the patients included and the different variables was carried out daily within the Influenza Epidemiological Surveillance Programme. Flu virus infection was detected by real-time reverse transcription polymerase chain reaction (RT-PCR) or rapid antigen (Ag) test in the microbiology laboratory from a clinical specimen of nasopharyngeal exudate or aspirate in patients with respiratory symptoms compatible with flu. The type of virus (A or B) was also determined.
Influenza vaccination status was obtained from the Spanish immunisation register. Those who had received a dose of flu vaccine at least 14 days before the onset of symptoms during the 2022/2023 season were considered vaccinated. The rest of the clinical/epidemiological variables collected were obtained from the electronic medical records. A patient was considered as having comorbidity when they had at least one of the following: cardiovascular disease, asthma, chronic obstructive pulmonary disease, diabetes mellitus, obesity, chronic kidney failure, pregnancy, liver disease or cancer. As with the criteria for defining severity, the presence or absence of these comorbidities was defined from the collected data according to the diagnoses in the medical history recorded by the clinician in charge of the patient in their electronic medical records.
Statistical analysisFirstly, we carried out a descriptive study on all patients included according to vaccination status and a comparison was made using the Chi-square test to check for differences between them. Then, to study the association between the development of severe flu and the different possible associated factors (flu vaccination, gender, age, comorbidities), the crude odds ratio (COR) and the adjusted odds ratio (AOR) were calculated using logistic regression. It was adjusted according to the variables which were significant in the descriptive study or in the calculation of the factors associated with the development of severe flu. Finally, the crude and adjusted vaccine effectiveness (VE) (for the prevention of severe cases of influenza) and its 95% confidence interval were calculated using the following formula: VE = (1-odds ratio) × 100. The calculation was carried out for both genders, age group and by presence of comorbidities.
The level of statistical significance used was P < .05. Statistical analysis was performed with the IBM® SPSS® Statistics software program, v. 25.0.
The study was approved by the Department of Health's Independent Ethics Committee for research with medicines (Ref.: 2019-044).
ResultsFrom week 40 of 2022 to week 20 of 2023, a total of 403 patients over the age of 6 months were admitted with influenza confirmed by RT-PCR. Of these, 313 (77.7%) were influenza A and 90 (22.3%) influenza B. Of all the patients included, 98 (24.3%) met at least one of the severity criteria.
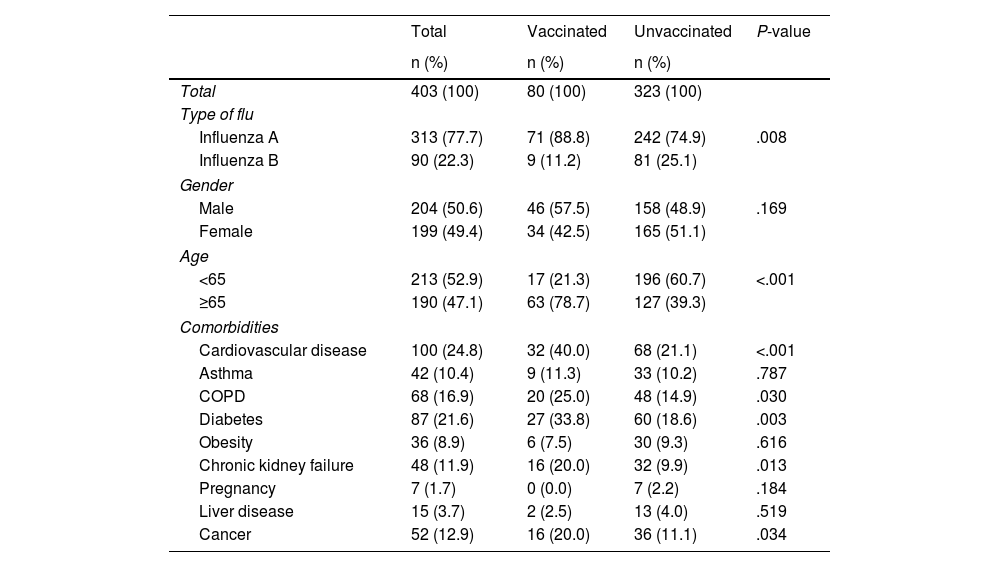
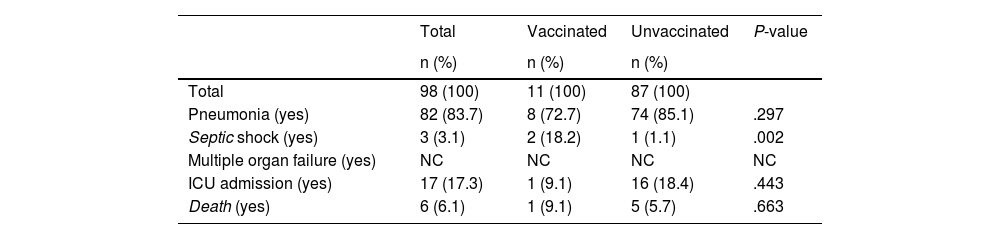
Of the 403 patients included, 80 (19.9%) had received the vaccine. Of the total number of patients, 287 had an indication to be vaccinated (71.2%): 190 because they were over the age of 65, with 63 of them having been vaccinated (33.2%); and 97 because, although under 65, they had some type of comorbidity, with 10 of them having been vaccinated (10.3%). The patients' characteristics are shown in Table 1. There were significant differences between the two groups (vaccinated and unvaccinated) in the type of flu (P = .008), age (P < .001) and having cardiovascular disease (P ≤ .001), COPD (P = .030), diabetes (P = .003), kidney failure (P = .013) and cancer (P = .034). No differences were found in terms of gender or other comorbidities. The characteristics of the patients with severe influenza are shown in Table 2. Of the 17 patients (17.3%) who required admission to the ICU, 16 were not vaccinated, and of the six who died (6.1%), five had not been previously vaccinated against the flu.
Characteristics of patients with influenza admitted from week 40/2022 to week 20/2023, according to flu vaccination status (n = 403).
| Total | Vaccinated | Unvaccinated | P-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Total | 403 (100) | 80 (100) | 323 (100) | |
| Type of flu | ||||
| Influenza A | 313 (77.7) | 71 (88.8) | 242 (74.9) | .008 |
| Influenza B | 90 (22.3) | 9 (11.2) | 81 (25.1) | |
| Gender | ||||
| Male | 204 (50.6) | 46 (57.5) | 158 (48.9) | .169 |
| Female | 199 (49.4) | 34 (42.5) | 165 (51.1) | |
| Age | ||||
| <65 | 213 (52.9) | 17 (21.3) | 196 (60.7) | <.001 |
| ≥65 | 190 (47.1) | 63 (78.7) | 127 (39.3) | |
| Comorbidities | ||||
| Cardiovascular disease | 100 (24.8) | 32 (40.0) | 68 (21.1) | <.001 |
| Asthma | 42 (10.4) | 9 (11.3) | 33 (10.2) | .787 |
| COPD | 68 (16.9) | 20 (25.0) | 48 (14.9) | .030 |
| Diabetes | 87 (21.6) | 27 (33.8) | 60 (18.6) | .003 |
| Obesity | 36 (8.9) | 6 (7.5) | 30 (9.3) | .616 |
| Chronic kidney failure | 48 (11.9) | 16 (20.0) | 32 (9.9) | .013 |
| Pregnancy | 7 (1.7) | 0 (0.0) | 7 (2.2) | .184 |
| Liver disease | 15 (3.7) | 2 (2.5) | 13 (4.0) | .519 |
| Cancer | 52 (12.9) | 16 (20.0) | 36 (11.1) | .034 |
COPD: chronic obstructive pulmonary disease.
Characteristics of patients with severe flu from week 40/2022 to week 20/2023, according to flu vaccination status (n = 98).
| Total | Vaccinated | Unvaccinated | P-value | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Total | 98 (100) | 11 (100) | 87 (100) | |
| Pneumonia (yes) | 82 (83.7) | 8 (72.7) | 74 (85.1) | .297 |
| Septic shock (yes) | 3 (3.1) | 2 (18.2) | 1 (1.1) | .002 |
| Multiple organ failure (yes) | NC | NC | NC | NC |
| ICU admission (yes) | 17 (17.3) | 1 (9.1) | 16 (18.4) | .443 |
| Death (yes) | 6 (6.1) | 1 (9.1) | 5 (5.7) | .663 |
NC: not calculable; ICU: Intensive care unit.
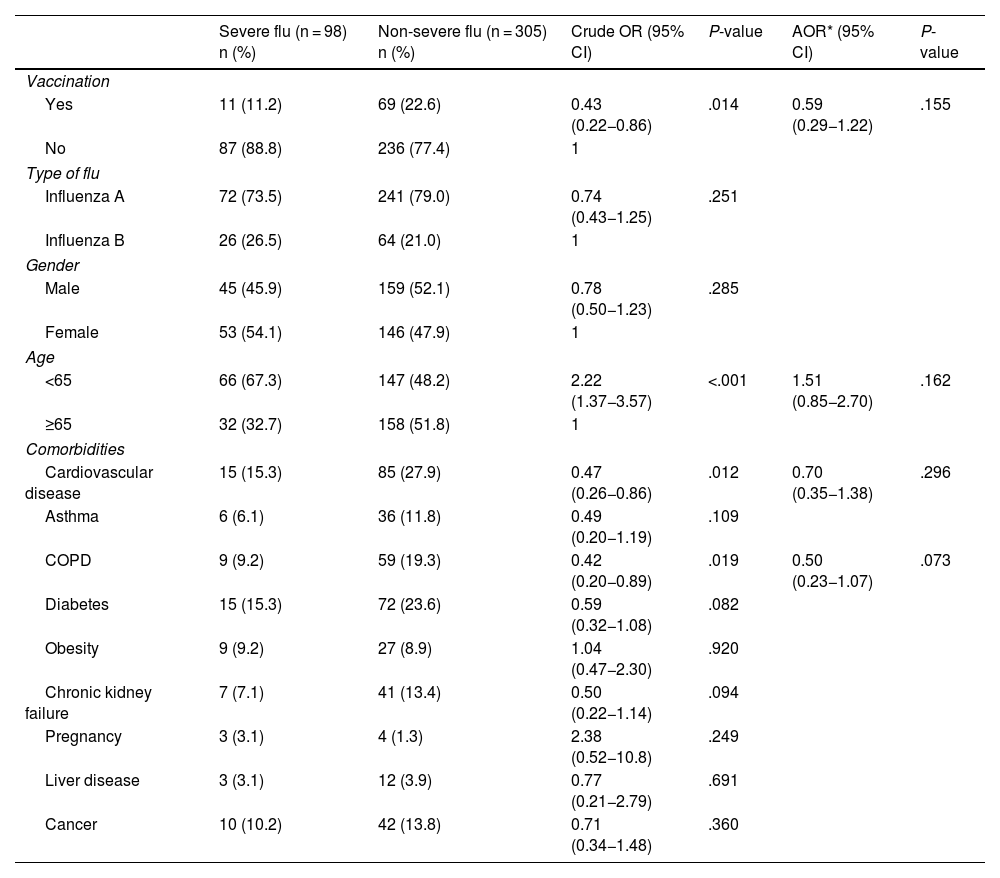
The vaccination rate in cases of severe flu was 11.2% (11/85) compared to 22.6% (69/305) in the group of patients with non-severe flu. Vaccination was independently associated as a protective factor with the development of severe flu (adjusted odds ratio [AOR] = 0.59; 0.29−1.22). The study of the factors associated with the development of severe flu is shown in Table 3.
Factors associated with the development of severe flu (n = 403).
| Severe flu (n = 98) n (%) | Non-severe flu (n = 305) n (%) | Crude OR (95% CI) | P-value | AOR* (95% CI) | P-value | |
|---|---|---|---|---|---|---|
| Vaccination | ||||||
| Yes | 11 (11.2) | 69 (22.6) | 0.43 (0.22−0.86) | .014 | 0.59 (0.29−1.22) | .155 |
| No | 87 (88.8) | 236 (77.4) | 1 | |||
| Type of flu | ||||||
| Influenza A | 72 (73.5) | 241 (79.0) | 0.74 (0.43−1.25) | .251 | ||
| Influenza B | 26 (26.5) | 64 (21.0) | 1 | |||
| Gender | ||||||
| Male | 45 (45.9) | 159 (52.1) | 0.78 (0.50−1.23) | .285 | ||
| Female | 53 (54.1) | 146 (47.9) | 1 | |||
| Age | ||||||
| <65 | 66 (67.3) | 147 (48.2) | 2.22 (1.37−3.57) | <.001 | 1.51 (0.85−2.70) | .162 |
| ≥65 | 32 (32.7) | 158 (51.8) | 1 | |||
| Comorbidities | ||||||
| Cardiovascular disease | 15 (15.3) | 85 (27.9) | 0.47 (0.26−0.86) | .012 | 0.70 (0.35−1.38) | .296 |
| Asthma | 6 (6.1) | 36 (11.8) | 0.49 (0.20−1.19) | .109 | ||
| COPD | 9 (9.2) | 59 (19.3) | 0.42 (0.20−0.89) | .019 | 0.50 (0.23−1.07) | .073 |
| Diabetes | 15 (15.3) | 72 (23.6) | 0.59 (0.32−1.08) | .082 | ||
| Obesity | 9 (9.2) | 27 (8.9) | 1.04 (0.47−2.30) | .920 | ||
| Chronic kidney failure | 7 (7.1) | 41 (13.4) | 0.50 (0.22−1.14) | .094 | ||
| Pregnancy | 3 (3.1) | 4 (1.3) | 2.38 (0.52−10.8) | .249 | ||
| Liver disease | 3 (3.1) | 12 (3.9) | 0.77 (0.21−2.79) | .691 | ||
| Cancer | 10 (10.2) | 42 (13.8) | 0.71 (0.34−1.48) | .360 | ||
95% CI: 95% confidence interval; COPD: chronic obstructive pulmonary disease.
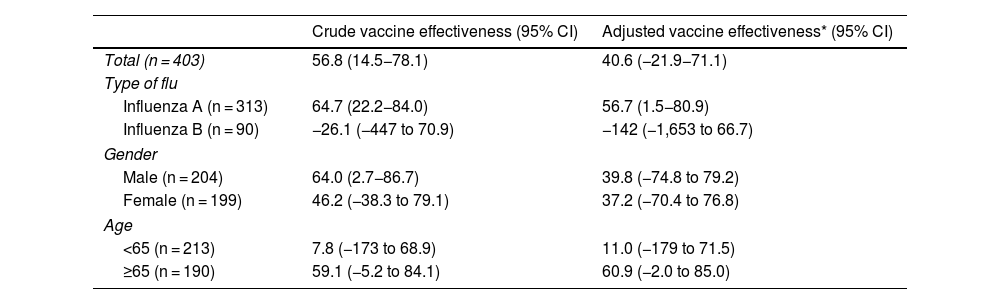
The crude VE in preventing cases of severe flu was 56.8% (14.5−78.1) and the VE adjusted by type of flu, age group, cardiovascular disease, COPD, diabetes, kidney failure and cancer was 40.6% (−21.9 to 71.1) (Table 4). In the adjusted analysis restricted to each type of flu, gender, age group and comorbidities, the flu vaccine was effective in preventing severe cases in all categories, and was particularly relevant in the over-65 age group (aVE = 60.9%; −2.0 to 85.0). For the prevention of influenza A, the VE remained significant when adjusted (aVE = 56.7%; 1.5−80.9).
Crude and adjusted flu vaccine effectiveness for the prevention of severe cases of flu restricted to both genders, age group and having comorbidities (n = 403).
| Crude vaccine effectiveness (95% CI) | Adjusted vaccine effectiveness* (95% CI) | |
|---|---|---|
| Total (n = 403) | 56.8 (14.5−78.1) | 40.6 (−21.9−71.1) |
| Type of flu | ||
| Influenza A (n = 313) | 64.7 (22.2−84.0) | 56.7 (1.5−80.9) |
| Influenza B (n = 90) | −26.1 (−447 to 70.9) | −142 (−1,653 to 66.7) |
| Gender | ||
| Male (n = 204) | 64.0 (2.7−86.7) | 39.8 (−74.8 to 79.2) |
| Female (n = 199) | 46.2 (−38.3 to 79.1) | 37.2 (−70.4 to 76.8) |
| Age | ||
| <65 (n = 213) | 7.8 (−173 to 68.9) | 11.0 (−179 to 71.5) |
| ≥65 (n = 190) | 59.1 (−5.2 to 84.1) | 60.9 (−2.0 to 85.0) |
COPD: chronic obstructive pulmonary disease; 95% CI: 95% confidence interval.
It has been estimated that the vaccine effectiveness in preventing cases of severe influenza, adjusted for the type of influenza, age group and the most significant comorbidities, was 40.6%. This signifies it has a very positive protective effect for the prevention of serious illness. With this estimate, it can be stated that vaccination prevented the development of serious symptoms in almost half of the patients admitted with already confirmed influenza infection. This effect was particularly maintained in groups in which vaccination was indicated, such as those aged over 65 and patients with any comorbidity.
Furthermore, such a positive effect was achieved despite low vaccination coverage, with 19.9% of the total sample (80/403) having received the vaccine. This not only shows the fundamental role of vaccination in preventing serious cases of flu, but also shows us that vaccine coverage is an obvious area for improvement. The protective effect achieved with vaccination is particularly relevant in patients in whom vaccination is indicated due to risk conditions (age or morbidity). It is in these groups that a better VE and a higher vaccination rate is achieved, although there is still room for improvement.
Calculating VE to prevent severe flu cases is not routine, so it is difficult to make comparisons. What can be compared are the results obtained here with those of a study with the same characteristics carried out in our own centre in the 2018/19 season.18 Although we see a slight decrease in VE, the effect continues to be maintained, and the morbidity and mortality rates among hospitalised patients has been considerably reduced. This difference may be due to multiple factors, some of them intrinsic to the patients themselves (greater comorbidity or complexity), or to less similarity between the circulating strains and this season's vaccine.
As there are few studies with the same methodology, the best option to evaluate our results may be to compare them with studies with similar patient samples. Similar studies would be those which analyse the impact of vaccination in hospitalised patients,19 given that their characteristics are similar to those included in our study.
Our findings are in line with those obtained in other earlier studies with hospitalised patients. Nevertheless, we have to bear in mind that these estimates can vary from one season to another, and even within the same season, depending on the results studied or the methodology applied.
In a study of the 2021/22 season, covering five different hospitals in Italy,20 which in turn was part of the Development of Robust and Innovative Vaccine Effectiveness (DRIVE) project, vaccine coverage of 42.8% was achieved and an overall VE of 83%–84% was obtained in hospitalised patients. In that study they did not break down their estimates according to age or comorbidities, and nor did they take into account the prevention of severe cases. They did report, however, that VE was possibly higher in terms of more serious clinical outcomes, such as admission to ICU and death, as, in cases where it did not prevent infection per se, it may have reduced the severity of the disease. We should highlight that in our study, of the 17 patients admitted to the ICU and the six who died, 16 and five respectively were not vaccinated.
This last reflection is consistent with what we were able to extract from another previous study14 carried out at 12 Spanish hospitals over six flu seasons (2010–2016) in which the adjusted VE for preventing admission or death in ICU was 23%. These criteria are more in line with those we used in our own analysis.
Despite these similarities, we see how in recent years, specifically in the seasons of 2018/1921 and 2021/22,22 some of the VE calculations decreased in these patient profiles. Once again, it is difficult to find one single reason, as the improvement seen in the current 2022/2023 season could well be due to both intrinsic characteristics of the patients and the strains included in the vaccine corresponding better to the strains circulating.
Nevertheless, having obtained such positive results, it would be worth recommending that the estimating of the severity of cases should become routine. This could be of great benefit in preventing hospital cases and outpatient cases by encouraging the population to get vaccinated. The possible improvement can be particularly appreciated in the preliminary results of this last season 2022/2323 in outpatients, where VE of 27% and 50% was achieved against influenza A and influenza B respectively. Adding these results to those from our study, we can conclude that effective influenza vaccination generates significant protection against influenza B infection and, although the same level of effectiveness is not obtained for influenza A, it would help to prevent the infection from progressing towards a more serious condition.
The limitations of our study are mainly due to the way the information was obtained, through electronic medical records and the Spanish immunisation register, meaning we were depending on third parties. Furthermore, despite differentiating the VE according to cases of influenza A or B, the sub-typing of each strain was not determined, so the specific VE in each strain could not be estimated as in other similar studies. We should also point out that our hospital's admission criteria may have biased the obtaining of controls, as these were patients with confirmed flu, but who did not meet the severity criteria which would have led to their hospital admission and to them therefore becoming part of our study sample. Lastly, we should stress that this study has some important strengths since, as previously mentioned, it not only takes into account admission to ICU or a patient's death to define severity in hospitalised patients, it also considers the development of pneumonia, septic shock or multiple organ failure as complications defining the seriousness of the case and which, in turn, increase healthcare costs and the burden of hospital morbidity and mortality.24
We can state that the vaccine continues to be the most effective preventive tool for facing the annual flu pandemics. Our results show that it is essential to continue pushing for annual vaccination of the highest-risk patients.
This study provides new evidence that high coverage in these patient groups should be a public health goal in order to considerably reduce the burden of morbidity and mortality, along with the associated costs, caused by influenza every year.
In view of this situation, we see it as a priority to rethink more ambitious strategies to help generate this much-needed increase in uptake, establishing new vaccination policies capable of attracting a greater number of patients, and particularly those who may be more vulnerable to complications if they contract the infection.
FundingThe project was submitted for the 10th Call for Grants to Support and Promote Research of the Instituto de Investigación Sanitaria y Biomédica de Alicante (ISABIAL) [Alicante Health and Biomedical Research Institute], with the title “Efectividad Vacunal frente a la gripe para prevenir los casos de gripe grave en la temporada 2022/2023” [Influenza vaccine effectiveness in preventing cases of severe flu in the 2022/2023 season]. It was approved with file number 2023-0117.
AuthorsGuillermo Platas Abenza, Pablo Chico Sánchez and Paula Gras Valentí participated in the conception and design of the study.
María Guerrero-Soler, Raissa de Fatima Silva Afonso, Pilar Gallardo Rodríguez, Francisco Gil Sánchez, Isabel Escribano Cañadas, Carmen M. Benito Miralles, Noemí Solis Aniorte, Rocío Carnicer Bueno and Ana Esclapez Martínez participated in the data collection.
José Sánchez Payá, Guillermo Platas Abenza, Pablo Chico Sánchez and Paula Gras Valentí performed the primary data analysis.
All the authors participated in the interpretation of results. Guillermo Platas Abenza, Pablo Chico Sánchez and Paula Gras Valentí participated in the preparation of the draft of the article. All the authors critically reviewed the intellectual content and approved the final version.
Conflicts of interestThe authors declare that they have no conflicts of interest.