Infections of cardiac electrical stimulation devices result in prolonged intravenous treatment with derived complications. In patients with cardiac electrical stimulation, bacteraemia due to Staphylococcus aureus is associated with high morbidity and mortality.1 Definitive antibiotic treatment should be based on the recommendations for endocarditis. Therapeutic failure is common.2
We present a case of bacteraemia due to methicillin-susceptible S. aureus (MSSA) in a patient fitted with an implantable cardioverter defibrillator (ICD) with septic thrombophlebitis of the internal jugular vein, treated with dalbavancin. This antibiotic offers dosage advantages and has demonstrated its activity in bacteraemia and in foreign body infections, although its use has still not been approved for these indications.
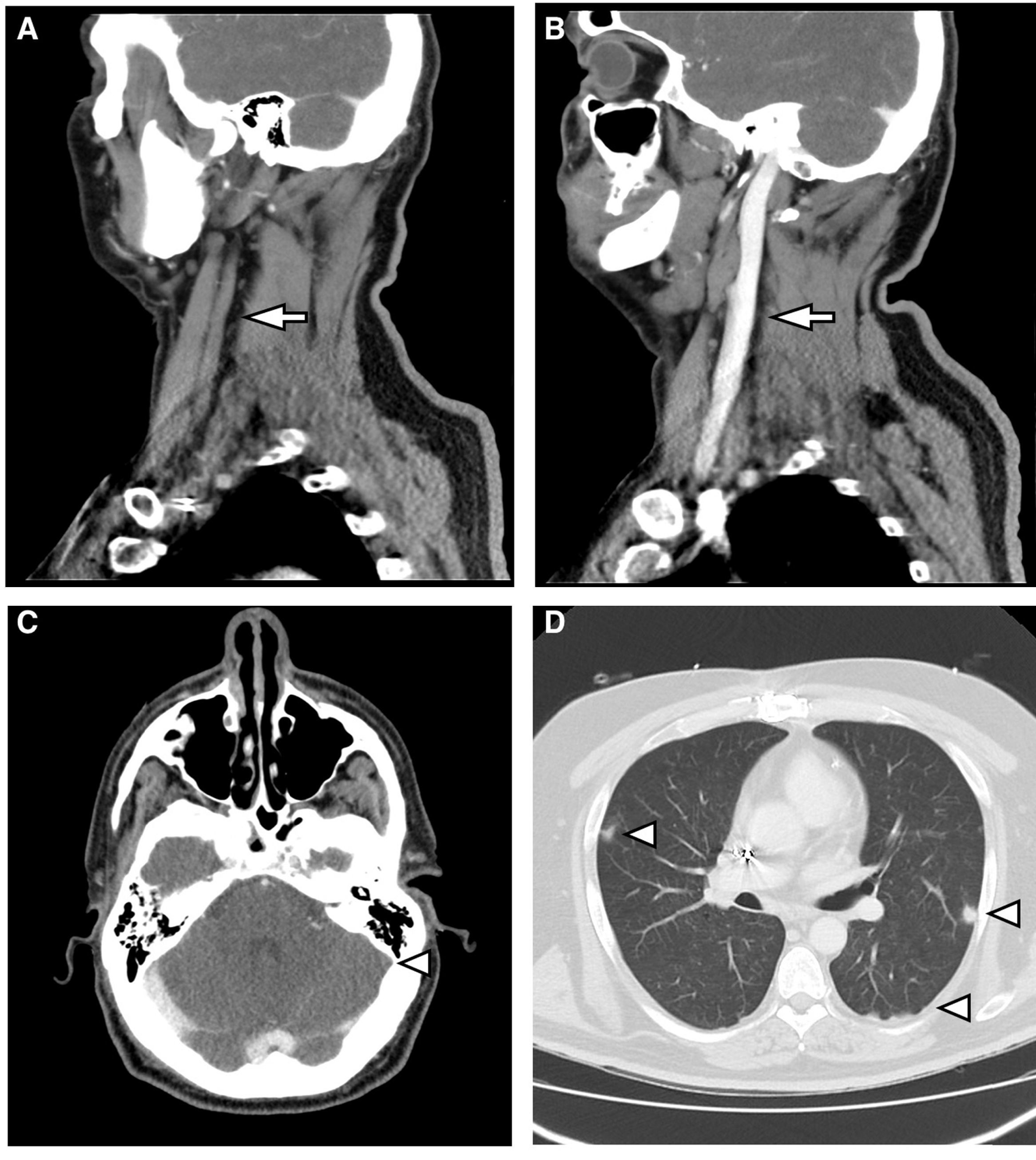
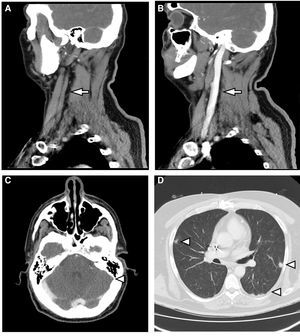
This case discusses a 46-year-old male who was hospitalised to investigate chest pain. He had a history of acute myocardial infarction which had occurred six months previously, and an ICD was placed through the left subclavian vein two weeks prior to this admission. On the second day of hospitalisation, he had an episode of fever at 39°C associated with phlebitis in the peripheral venous catheter. Treatment was started with amoxicillin-clavulanic acid. After the first dose, having observed growth of MSSA in 2/2 blood cultures, 2g of intravenous cloxacillin was established and repeated every 6h. A transthoracic and transoesophageal echocardiogram was performed, which did not show endocarditis or infection of the ICD lead. Given bacteraemia due to S. aureus, assessment of the removal of the device was requested by Cardiology. The removal was initially rejected as the procedure was deemed to be too risky to be undertaken at our centre. After three days of antibiotic therapy, the fever persisted and MSSA was isolated again in the blood culture. Therefore, 10mg/kg of daptomycin every 24h was added to the previous treatment. A computerised axial tomography of the cervical-thoracic region was requested. This showed findings compatible with thrombosis of the left internal jugular vein which extended to the sigmoid sinus and bilateral pulmonary septic emboli (Fig. 1). It was decided to discontinue cloxacillin and start cefazolin (2g/8h) to minimise manipulation of the venous catheter. Treatment with daptomycin was maintained. After three days of treatment, the patient presented with a new episode of phlebitis and it was impossible to channel new peripheral venous access. Due to the need for intravenous antibiotic therapy for six weeks, given the possibility of infection of the ICD lead, and considering the difficulty maintaining venous access, it was decided to discontinue treatment and start 1500mg of dalbavancin every two weeks, for six weeks in total. This was administered on an outpatient basis, with excellent clinical course and blood tests. Control blood cultures were requested up to week 12 post-treatment, which were sterile, and a PET-CT scan was performed at the end of treatment, with no bacterial collection in the lead or in the heart valves, with reduction in the size of the thrombosis and complete resolution of the lung lesions.
(A) Sagittal reconstruction of MDCT with intravenous contrast. Absence of flow in the left internal jugular vein (arrow). (B) It is compared with the well-filled contralateral internal jugular vein (arrow). (C) MDCT with intravenous contrast. Absence of flow in sagittal sinus (arrowhead). (D) MDCT with lung window. Multiple parenchymal nodules of peripheral distribution are observed (arrowheads).
Dalbavancin is a lipoglycopeptide approved for the treatment of skin and soft tissue infections. It offers dosage advantages given its prolonged half-life,3 allows for weekly intravenous administration, and there are studies which show similar efficacy in single-dose regimens of 1500mg.4 Although the only indication approved by the FDA/EMA is for skin and soft tissue infections, there are data which show its activity at other levels. A phase 2 clinical trial on patients with catheter-related bacteraemia revealed an overall success rate for dalbavancin greater than that for vancomycin (87 vs 50%).5 There are studies in animal models of endocarditis due to S. aureus which show that dalbavancin has greater activity than teicoplanin and vancomycin,6 and other studies show its activity in foreign body infections due to S. aureus7 and in subcutaneous device-associated infections.8 Recent studies demonstrate efficacy in in vitro reduction of biofilms to concentrations that may be obtained easily in vivo,9 which supports the previous findings published on the likely potential of this drug in the treatment of device-associated infections. In the case of our patient, in whom, exceptionally, the device was not removed, dalbavancin has proven to be effective at controlling the infection, with no adverse effects. Thanks to the regimen used, the risk and healthcare cost, which a prolonged admission would have involved, were avoided.
The probable role that this antibiotic may play in intravascular and foreign body infections is clear. This opens up new therapeutic options in the outpatient setting, although studies are needed to confirm this hypothesis.
Please cite this article as: Martínez-Sanz J, Gijón de la Santa L, Torralba M. Tratamiento con dalbavancina en paciente con tromboflebitis séptica yugular por Staphylococcus aureus tras la inserción de un desfribilador automático implantable. Enferm Infecc Microbiol Clin. 2018;36:389–390.