To characterize a methicillin-resistant Staphylococcus aureus (MRSA) isolate responsible for an aggressive infection (peridural and psoas abscess secondary to haematogenous septic arthritis) in a poultry farmer.
MethodsMolecular characterization was performed, including spa- and multilocus sequence typing of the isolate, assessment of its resistance phenotype and detection of tetracycline resistance and of virulence and immune evasion cluster (IEC) genes were performed.
ResultsThe MRSA isolate was tetracycline- and fluorquinolone-resistant, and was ascribed to CC398, spa-t1451. The isolate harboured tet(M) (distinctive of livestock-associated (LA) MRSA-CC398 clade) and IEC-type B system (characteristic of the methicillin-susceptible human lineage, but typically absent in LA-MRSA-CC398 strains), and lacked toxin-coding genes lukF/lukS-PV, tsst-1, eta and etb.
ConclusionIEC re-acquisition by LA-MRSA-CC398-LA strains is an unusual finding, but could constitute an emerging public health problem. It would represent an evolutionary step towards LA-MRSA-CC398's adaptation to human hosts, and might enhance its invasiveness and ability to be transmitted to humans.
Caracterizar un aislado de Staphylococcus aureus resistente a meticilina (SARM), causante de una infección muy agresiva (absceso epidural y de psoas secundarios a artritis séptica hematógena) en un granjero avícola.
MétodosEl aislado fue caracterizado molecularmente (spa- y multilocus sequence typing), y se estudió su fenotipo de resistencia y la presencia de genes de resistencia a tetraciclina, de virulencia y del sistema immune evasion cluster (IEC).
ResultadosEl aislado de SARM, resistente a tetraciclina y fluoroquinolonas, fue tipado como spa-t1451-CC398, albergaba el gen tet(M) (distintivo de SARM-CC398 asociado al ganado [AG]) y el sistema IEC-tipo B (característico de S. aureus meticilin-sensible-CC398 adscrito al clado humano, pero no de SARM-CC398-AG), carecía de lukF/lukS-PV, tsst-1, eta, y etb.
ConclusiónLa readquisición del sistema IEC por aislados SARM-CC398-AG es excepcional, pero constituiría un problema emergente de salud pública. Representaría un paso evolutivo en la readaptación de SARM-CC398-AG al hombre, pudiendo incrementar su invasividad y transmisibilidad a humanos.
In the early 2000s, livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) belonging to clonal complex (CC) 398 emerged as a zoonotic pathogen. MRSA-CC398 was able to colonize and infect people exposed to farm-animals (particularly to pigs),1 although it has later been isolated from humans with no documented livestock contact.2,3 Phylogenetic analyses demonstrate that MRSA-CC398 originated in humans as methicillin-susceptible and that mecA acquisition occurred several times after its introduction to livestock. This jump between species was accompanied by further genomic changes that enhanced its adaptation to animal hosts, such as the acquisition of Tn916-like – which harbours tetracycline resistance gene tet(M) – and the loss of ΦSa3 prophage, which carries the immune evasion gene cluster (IEC) that facilitates human colonization and invasion.1 MRSA-CC398 usually exhibits low virulence and transmissibility capacity compared to other community-acquired MRSA clonal lineages, such as ST8 or ST80.4,5 Even though MRSA-CC398 has been mainly described in human colonization and in respiratory and mild skin infections,6,7 it has been also identified as the etiological agent of more serious invasive infections, e.g. endocarditis, osteomyelitis, cellulitis, or pyomyositis.6
We report a case of peridural and psoas abscess secondary to septic arthritis of lumbar facet joint, caused by a MRSA-CC398 isolate carrying tet(M) and IEC type B genes, in a poultry-farm worker.
Material and methodsClinical caseA 49-year-old obese male presented to the emergency department with a history of persistent lower back pain accompanied by fever. The anamnesis revealed that two months earlier he had suffered a toe wound, which became infected and healed after oral amoxicillin-clavulanate treatment, and that he was the owner of a poultry-farm.
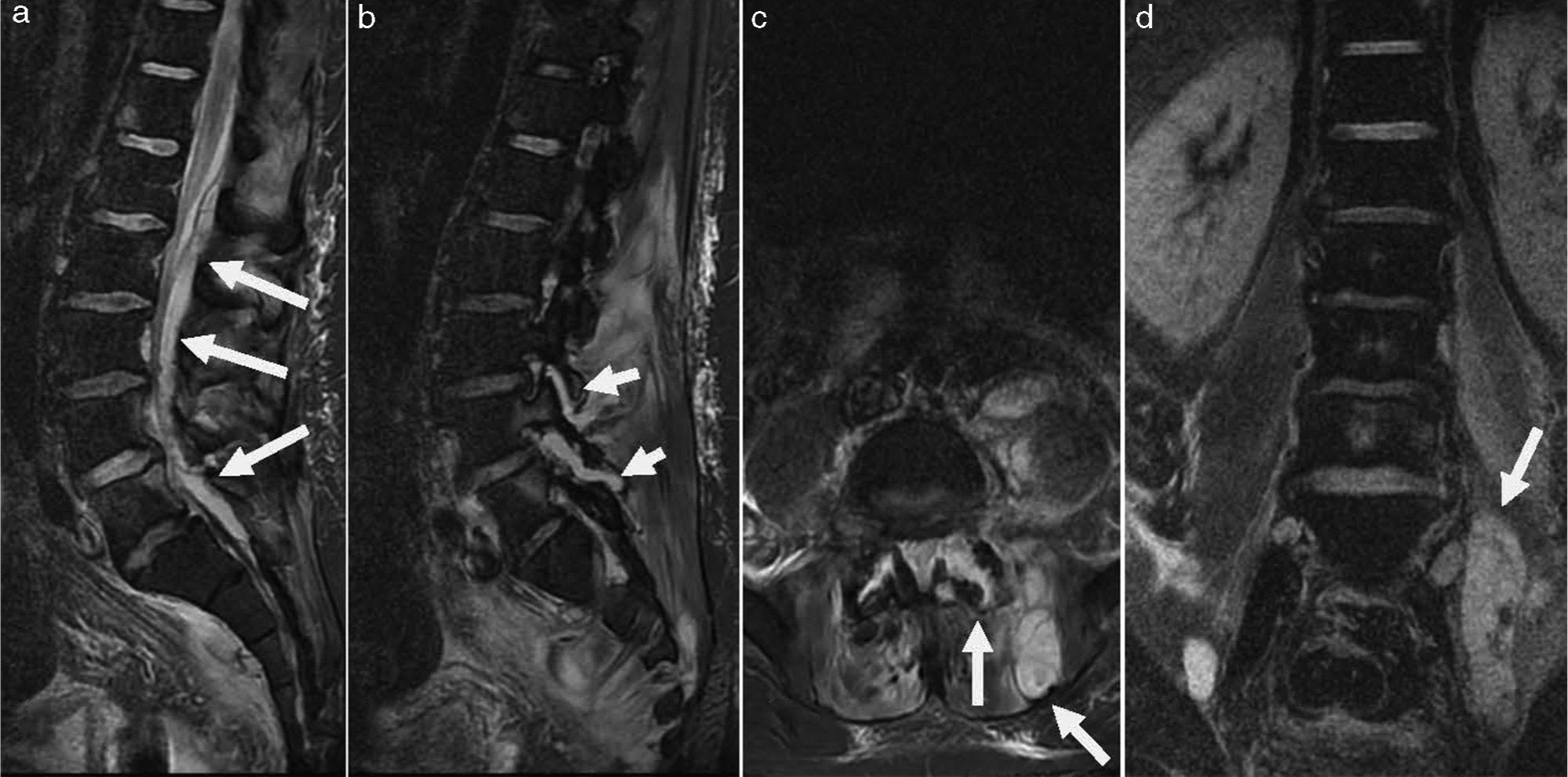
On clinical examination, he had high temperature (39.5°C), L5 back pain with signs of radicular affectation and a bladder globus. Haematological examination revealed raised C-reactive protein (233mg/dL; reference interval <10mg/dL) and neutrophil leucocytosis (24.4×109L−1). An urgent abdominopelvic and lumbar CT scan showed a psoas abscess and affectation of L4-L5 facet joint. The patient was admitted to the traumatology ward and, despite been commenced on intravenous amoxicillin-clavulanate therapy, his neurological condition worsened with increasing pain and symptoms of Cauda Equina syndrome. A lumbar magnetic resonance imaging demonstrated a large psoas abscess, a D12-S1 peridural abscess and a purulent collection in L4-L5 facet joint (Fig. 1). The patient underwent surgical abscess drainage and, subsequently, his clinical condition improved.
Urine and blood samples, collected at the emergency department, as well as pus cultures grew MRSA. Antibiotic treatment was changed, according to susceptibility results, to intravenous daptomycin and clindamycin. Surveillance cultures demonstrated nasal and perineal MRSA carriage.
Four days after surgery, the patient's clinical condition deteriorated again with relapse of febrile illness and abdominal and groin pain; hence, CT-guided percutaneous drainage of the psoas abscess was undertaken, yielding 130ml of purulent material. The following days the patient recovered progressively and remission of fever and neurological symptoms was achieved. Intravenous antimicrobial therapy was continued for six weeks, after which the patient was discharged home with a regime of oral rifampicin and co-trimoxazole until complete normalization of acute-phase reactants, which took place after 4 weeks. Evolution was favourable with complete resolution of the abscesses, absence of neurological sequelae and recovery of bladder and sphincters function.
Microbiological methodsUrine and blood samples, pus from abscesses, and perineal and nasal swabs were processed at the microbiology laboratory following the standard procedures. Species identification and antibiotic susceptibility testing were carried out on the MicroScan system (Siemens Healthcare Diagnostics), and results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing guidelines (EUCAST) (http://www.eucast.org).
MRSA strains were characterized by sequence-based typing of the hyper-variable region of the S. aureus protein A (spa) gene2 and sequences were analysed using the Ridom Staph-Type software version 1.5.21 (Ridom GmbH). Multi-locus-sequence-typing (MLST) was performed as indicated (http://www.saureus.mlst.net).
The presence of tetracycline resistance genes tet(K), tet(L), and tet(M), and of the genetic determinants of the Panton-Valentine leukocidin (PVL) (lukF/lukS-PV), toxic shock syndrome toxin (tsst-1), and exfoliative toxin A (eta), and B (etb) was investigated by PCR.2 The genes included in the IEC (scn, chp, sak, sea and/or sep) were studied by PCR, and strains were classified, according to the pattern of genes detected, into one of the five IEC types.2 Moreover, specific mobile genetic elements associated with poultry strains were studied as previously reported.8
ResultsUrine, blood, pus and nasal and perineal swabs cultures yielded MRSA isolates, that were phenotypically and genotypically identical to each other. These isolates were resistant to tetracycline, ciprofloxacin and levofloxacin and remained susceptible to erythromycin, clindamycin, glycopeptides, linezolid, daptomycin, co-trimoxozole, fosfomycin, amikacin, gentamicin, tobramycin, mupirocin, and fusidic acid. Genetic analysis revealed that the MRSA strain belonged to spa-type t1451, CC398, and that it carried tet(M), but not tet(K) or tet(L). In addition, it harboured chp, sak and scn genes and, hence, was typed as IEC type B. However, no lukF/lukS-PV, tsst-1, eta, etb, or mobile genetic elements associated with poultry were detected.
DiscussionHere we have described a case of a very aggressive infectious process (peridural and psoas abscess secondary to haematogenous septic arthritis of lumbar facet joint, being the presumptive source of infection a toe wound) due to a CC398 spa t1451 MRSA strain, in a patient with occupational exposure to poultry. Interestingly, this MRSA-CC398 strain exhibited features of both livestock-associated and human-associated clades.
MRSA-CC398 isolates were first reported in The Netherlands and France as colonizers of pigs and swine farmers, among whom they are widely disseminated,1 although they do not seem to be particularly host-specific and are able to colonize and infect other animal species (cattle, horses, dogs, poultry) and humans.9
In the current clinical case, given the patient's occupation and the detection of tet(M) in the MRSA-CC398 strain responsible for the infectious process, the isolate might be ascribed to the animal-associated clade. The presence of tet(M) is a distinctive characteristic of isolates belonging to this lineage, displaying a very high discriminatory ability, close to 100%.1,10 The acquisition of tetracycline resistance by the most ancestral CC398 human-associated strains, once introduced to livestock, might have provided them with a selective advantage under antibiotic selective pressure posed by the wide use of this antimicrobial in food-animals.1 Our isolate presented additional resistance to ciprofloxacin, a fact that, as well as erythromycin-, clindamycin- and multidrug-resistance, is very common among MRSA-CC398 isolates recovered in Spain.2
Two recent studies found a remarkable increase over the last years in the prevalence of MRSA-CC398 isolates infecting or colonizing patients in Spanish hospitals, at the expense of the livestock clade.2,7 Moreover, despite demonstrating that contact with animals (mainly pigs and poultry) was a significant risk factor for becoming colonized or infected by these strains, a considerable percentage of patients lacked animal exposure.2
As a rule, isolates belonging to MRSA-CC398, even when involved in serious or invasive infections as the one we report, possess less virulence factors than those ascribed to other clonal complexes and, as occurred in the isolate under discussion, they are devoid of genes encoding enterotoxins, toxic-shock-syndrome toxin, exfoliative toxins or Panton-Valentine leukocidine.11,12 On the other hand, our MRSA-CC398 strain, unlike those analysed in Benito et al. series,2 carried the Φ3-associated IEC type B, harbouring chp, sak and scn genes. These genes codify for a number of immune-modulating proteins that counteract human innate immunity: chp encodes a chemotaxis inhibitory protein; sak encodes a staphylokinase responsible for tissue destruction and scn encodes an efficient inhibitor of the complement pathway that prevents opsonophagocytosis and killing of S. aureus by human neutrophils. The IEC genes have been found almost exclusively in methicillin-susceptible S. aureus CC398 invasive isolates attributed to the ancient human clade, suggesting that they might play an important role in long-term colonization and invasive illness in humans, but would not be necessary to other hosts; indeed, loss of human-niche-specific genes, such as IEC genes, may be a result of adaptation to nonhuman hosts.1,13 The presence of IEC genes in MRSA-CC398 livestock lineage, although still very infrequent, has already been described, both from isolates of animal origin10,14 and from isolates producing nasal colonization or infections, including septicaemia, in humans.15 Reacquisition of IEC by the livestock-associated clade suggests a process of re-adaptation to human host and may constitute an emerging public health problem, since it may enhance its invasiveness and ability to be transmitted to humans, without affecting its adaptation to livestock hosts.15,16
Conflict of interestThe authors declare no conflict of interest.
C. Lozano had a contract associated with Project SAF2012-35474 during the experimental part of this work.
We are grateful to Lucy Dougherty for editorial assistance.