Bicuspid aortic valve (BAV) is the most frequent congenital cardiac disease. It is associated to a higher risk of cardiovascular complications, including infective endocarditis (IE).
MethodsRetrospective, observational and single centre study that included all patients with IE diagnosed between 1996 and 2014. An analysis was made of the epidemiological, clinical, microbiological and echocardiographic data, complications during hospital admission, need for surgery, in-hospital mortality, and 1-year follow-up. Cases with endocarditis on prosthetic valves or other locations were excluded, as well as those for which the aortic valve morphology had not been accurately defined. A comparative statistical analysis was performed between BAV and tricuspid (TAV).
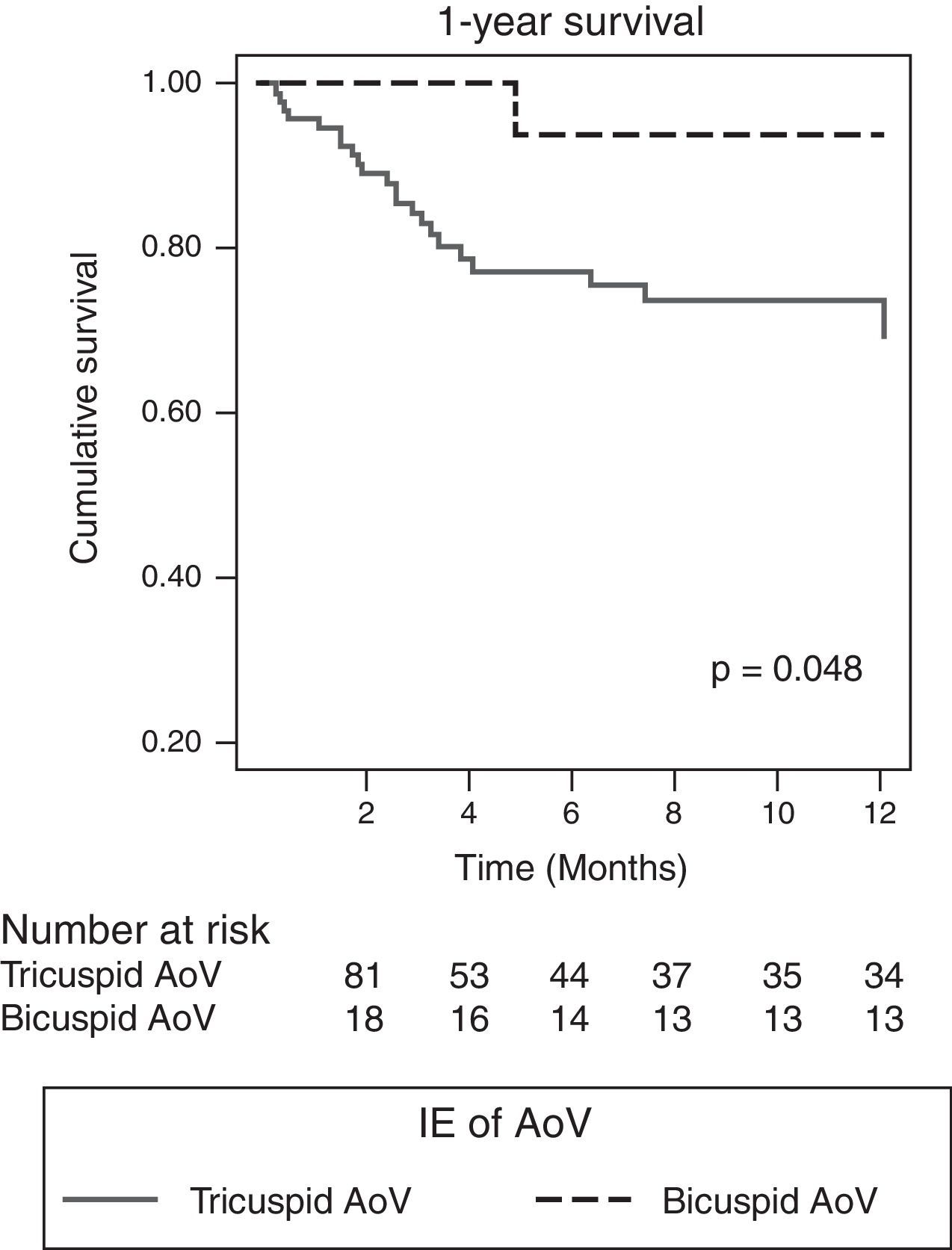
ResultsOf a total of 328 cases with IE, 118 (35.67%) were on aortic valve, with 18 (16.22%) of them being BAV. The BAV cases were younger than TAV (51±19.06 vs 60.83±15.73 years, p=0.021) and they had less comorbidity (Charlson 0.67±0.77 vs 1.44±1.64, p=0.03).). There was a higher tendency of staphylococcal origin (38.9 vs 21.5%, p=0.137), and 55.6% showed peri-valvular complications (TAV 16.1%, p=0.001), in particular, abscesses (38.9 vs 16.1%, p=0.047). BAV was the only predictive factor of peri-valvular complications (OR 7.87, 95% CI; 2.38–26.64, p=0.001). Patients with BAV had more surgery during their admission (83.3 vs 44.1%, p=0.004), had less in-hospital mortality, with no statistical significance (5.6 vs 25.8%, p=0.069), and 1-year survival was significantly superior (93.8 vs 69.3%, p=0.048).
ConclusionsPatients with IE on BAV are young, with low comorbidity. They frequently present with peri-valvular complications and they often require early surgery. Compared to TAV cases, in-hospital mortality is lower and 1-year survival is significantly higher.
La válvula aórtica bicúspide (VAB) es la malformación cardíaca congénita más frecuente. Se asocia a un mayor riesgo de complicaciones cardiovasculares, entre las que se incluye la endocarditis infecciosa (EI).
MétodosEstudio observacional, unicéntrico de cohorte, que incluye de forma prospectiva a todos los pacientes ingresados por EI entre 1996 y 2014. Se analizan datos epidemiológicos, clínicos, microbiológicos, ecocardiográficos, complicaciones durante la hospitalización, necesidad quirúrgica, mortalidad intrahospitalaria y seguimiento a un año. Se excluyen los casos con endocarditis sobre válvulas protésicas o en otras localizaciones, y aquellos de cuya válvula aórtica no se tienen datos certeros acerca de su morfología. Se ha realizado un análisis estadístico comparativo entre VAB y tricúspide (VAT).
ResultadosDe un total de 328 casos con EI, 118 (35,67%) fueron sobre válvula aórtica. Tenían VAB 18 (16,22%). Los casos con VAB eran más jóvenes que los portadores de VAT (51±19,06 vs. 60,83±15,73 años, p=0,021) y tenían menos comorbilidad (índice de Charlson 0,67±0,77 vs. 1,44±1,64, p=0,03). En el grupo con VAB observamos tendencia a EI causada por Staphylococcus spp. (38,9 vs. 21,5%, p=0,137). Con diferencia estadística, hubo más complicaciones perivalvulares entre los casos con VAB (55,6% vs. 16,1%, p=0,001) predominando los abscesos (38,9 vs. 16,1%, p=0,047). Ser portador de VAB fue el único factor predictor de las mismas (OR 7,87, IC del 95%, 2,38-26,64, p=0,001). Los pacientes con VAB se operaron más (83,3 vs. 44,1%, p=0,004) y la mortalidad durante el ingreso hospitalario fue menor, aunque no alcanzó significación estadística (5,6 vs. 25,8%, p=0,069). La supervivencia a un año fue significativamente superior en el grupo de VAB (93,8 vs 69,3%, p=0,048).
ConclusionesLos pacientes con EI sobre VAB son jóvenes, con poca comorbilidad asociada. Tienen frecuentemente complicaciones perivalvulares por lo que requieren cirugía precoz. La mortalidad intrahospitalaria comparada con EI sobre VAT es menor y la supervivencia a un año es significativamente mayor.
Bicuspid aortic valve (BAV) is the most common congenital heart defect. According to the literature, it has a prevalence of 1–2%,1,2 although data suggest that the figure is probably slightly lower in the Mediterranean population.3–5 It is known that these patients have a higher rate of cardiovascular complications, such as aortic valve (AoV) dysfunction,6–8 ascending aortic aneurysm,9–11 aortic dissection12,13 and infective endocarditis (IE).14
Few previous studies evaluate the clinical and echocardiographic characteristics and prognosis of patients with BAV who develop IE.
The objective of our study was, therefore, to determine these characteristics and identify differences with respect to IE of the tricuspid AoV (TAV).
MethodsStudy population and scopeObservational, single-centre cohort study, in which all patients admitted to a tertiary referral hospital for IE between January 1996 and December 2014 were included prospectively. We should point out that, from 2008 on, these patients were treated through a multidisciplinary alert strategy,15 as now recommended in the latest published guidelines.16 Patients with endocarditis in prosthetic valves or other locations, and those for whom there was no accurate AoV morphology data, were excluded from the analysis. An analysis was made of the epidemiological, clinical, microbiological and echocardiographic data, complications during hospital admission, need for surgery, in-hospital mortality, and 1-year follow-up.
Definition of study variablesClinical studyThe diagnosis of IE was considered when the modified Duke criteria were met.17 Comorbidity was assessed using the Charlson Comorbidity Index.18 Acute kidney injury (AKI) was defined by creatinine greater than 1.5mg/dl in patients with prior normal renal function or if there was >25% deterioration from baseline creatinine clearance in patients with chronic kidney disease (CKD). Heart failure was diagnosed according to the Framingham criteria and was considered severe when intravenous inotropic therapy or mechanical ventilation was required. Septic shock was defined according to the usual standards.19 Central nervous system conditions analysed include stroke (ischaemic or haemorrhagic) diagnosed after clinical examination and imaging tests, and encephalopathy, meningitis and brain abscess.
Early surgical treatment was defined as that performed during the hospital stay. The selection of patients considered for early surgical treatment vs medical management was made in each case through the agreement of infectious diseases specialists, cardiologists and cardiac surgeons according to internationally recognised recommendations.20 Time to surgery was taken as the time from the hospital admission date to the day of surgery. The EuroScore21 logistical scale was used to calculate the potential surgical risk. In-hospital mortality was considered as that occurring during admission or within 30 days after discharge, and mortality rates at 12 months after hospital discharge were also analysed.
Microbiological studyThis involved blood cultures (BACTEC® automated system) and culture of valve or other material obtained during surgery. From 2008, the polymerase chain reaction (16S rRNA universal PCR) on surgical specimens was also performed in cases where blood cultures were negative or doubtful in terms of IE aetiology. Blood cultures were performed at the end of treatment and two months later to confirm that the infection had been cleared.
Echocardiographic studyAll patients had a transthoracic echocardiogram, which included M-mode, two-dimensional (2D), spectral Doppler and colour images. A transoesophageal study was performed in all patients with a high suspicion of endocarditis who had a negative or dubious transthoracic study, and in those with suspected peri-valvular complications.22 The transoesophageal probes used were either monoplanar or biplanar until 2008, and multiplanar thereafter. The investigations were either performed in the echocardiography laboratory, in the critical area when the patient was haemodynamically unstable or required mechanical ventilation, or in theatre intraoperatively.
The degree of valvular regurgitation was assessed and classified as mild, moderate or severe, according to the American Society of Echocardiography guidelines.23
Vegetation was diagnosed when images of mobile mass were found on valve structures or an impact zone from abnormal jets with vibration or erratic movement different from that of the valve cusps where they settle. Abscess was defined as the presence of hypoechogenic zones or areas of irregular echogenicity with no evidence of flow within the area in the vicinity of the affected valve. Pseudoaneurysms were diagnosed by the presence of anechoic zones with internal flow and systolic expansion. Abnormal communications between two cardiac chambers were called fistulae.
BAV was diagnosed either by echocardiography (transthoracic or transoesophageal) or, in the case of patients with a poor acoustic window or with more calcified and unstructured valves, by direct visualisation by the cardiac surgeon.
Statistical analysisAll variables were compared between the two groups, IE of BAV and IE of TAV. The qualitative variables are expressed in percentages and the comparisons were analysed by means of the Chi-square test (χ2) or Fischer's exact test. Quantitative variables are expressed as mean±standard deviation; their distribution was analysed by the Kolmogorov–Smirnov test and the differences by Student's t test for variables following a normal distribution or the Mann–Whitney U test for those not following a normal distribution. The multivariate analysis was performed using binary logistic regression. We used the Kaplan–Meier method and Cox regression for the analysis of survival. All statistical analyses were carried out using Stata 13.1 (Statacorp, Texas, USA).
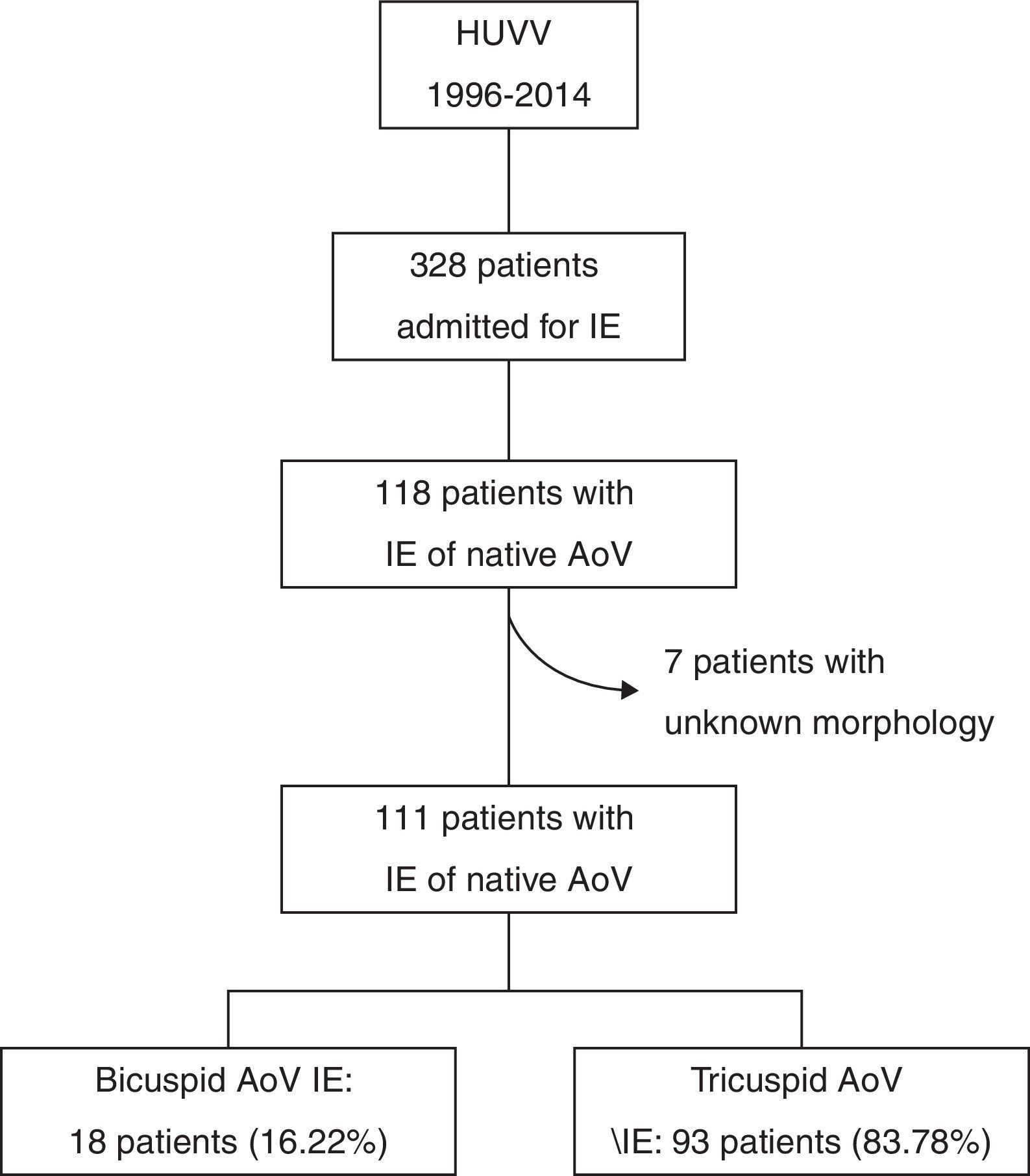
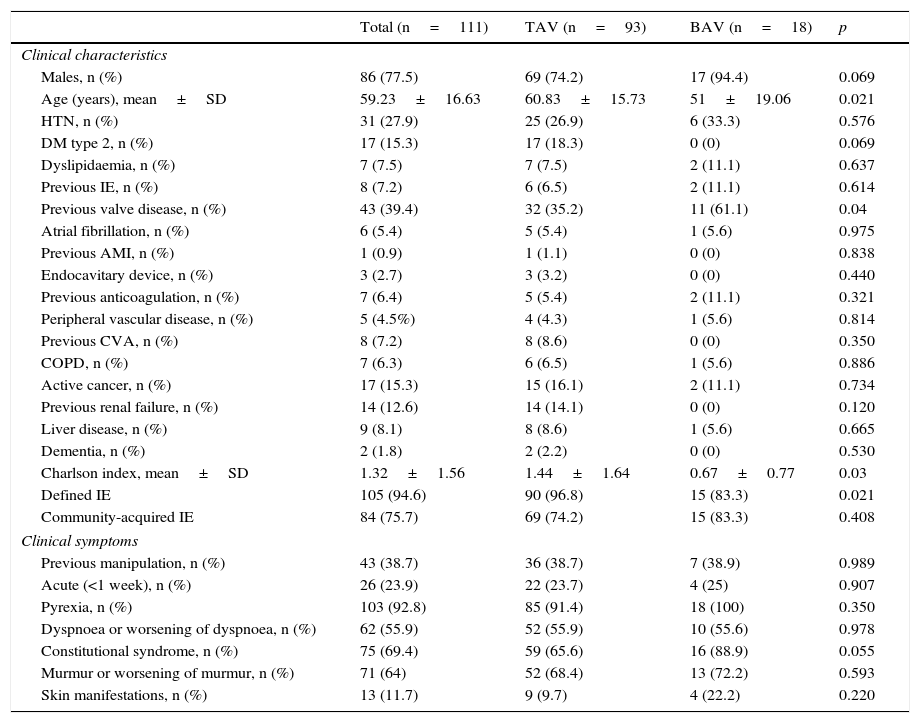
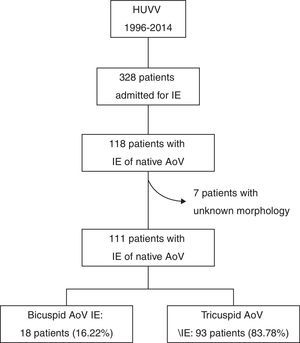
ResultsBaseline characteristicsOf the 328 admissions for IE during the study period, 118 were of native AoV (35.67%). There were no data recorded on AoV morphology for seven of these cases. Of the remaining 111, 18 (16.22%) had BAV (15 identified by echocardiogram and three by a cardiac surgeon during valve replacement) (Fig. 1). Compared to the population with TAV IE (n=93), these patients were significantly younger (51±19.06 vs 60.83±15.73 years, p=0.021) and they had less comorbidity (Charlson index 0.67±0.77 vs 1.44±1.64, p=0.03). According to modified Duke criteria, 94.6% of patients had “defined IE”, with the rate being higher in the TAV group (83.3% vs 96.8, p=0.021). A similar proportion of each group had community-acquired IE (83.3% vs 74.2%, p=0.408), and the prevalence of acute IE and other clinical findings was also similar (Table 1).
Baseline characteristics and clinical symptoms.
| Total (n=111) | TAV (n=93) | BAV (n=18) | p | |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Males, n (%) | 86 (77.5) | 69 (74.2) | 17 (94.4) | 0.069 |
| Age (years), mean±SD | 59.23±16.63 | 60.83±15.73 | 51±19.06 | 0.021 |
| HTN, n (%) | 31 (27.9) | 25 (26.9) | 6 (33.3) | 0.576 |
| DM type 2, n (%) | 17 (15.3) | 17 (18.3) | 0 (0) | 0.069 |
| Dyslipidaemia, n (%) | 7 (7.5) | 7 (7.5) | 2 (11.1) | 0.637 |
| Previous IE, n (%) | 8 (7.2) | 6 (6.5) | 2 (11.1) | 0.614 |
| Previous valve disease, n (%) | 43 (39.4) | 32 (35.2) | 11 (61.1) | 0.04 |
| Atrial fibrillation, n (%) | 6 (5.4) | 5 (5.4) | 1 (5.6) | 0.975 |
| Previous AMI, n (%) | 1 (0.9) | 1 (1.1) | 0 (0) | 0.838 |
| Endocavitary device, n (%) | 3 (2.7) | 3 (3.2) | 0 (0) | 0.440 |
| Previous anticoagulation, n (%) | 7 (6.4) | 5 (5.4) | 2 (11.1) | 0.321 |
| Peripheral vascular disease, n (%) | 5 (4.5%) | 4 (4.3) | 1 (5.6) | 0.814 |
| Previous CVA, n (%) | 8 (7.2) | 8 (8.6) | 0 (0) | 0.350 |
| COPD, n (%) | 7 (6.3) | 6 (6.5) | 1 (5.6) | 0.886 |
| Active cancer, n (%) | 17 (15.3) | 15 (16.1) | 2 (11.1) | 0.734 |
| Previous renal failure, n (%) | 14 (12.6) | 14 (14.1) | 0 (0) | 0.120 |
| Liver disease, n (%) | 9 (8.1) | 8 (8.6) | 1 (5.6) | 0.665 |
| Dementia, n (%) | 2 (1.8) | 2 (2.2) | 0 (0) | 0.530 |
| Charlson index, mean±SD | 1.32±1.56 | 1.44±1.64 | 0.67±0.77 | 0.03 |
| Defined IE | 105 (94.6) | 90 (96.8) | 15 (83.3) | 0.021 |
| Community-acquired IE | 84 (75.7) | 69 (74.2) | 15 (83.3) | 0.408 |
| Clinical symptoms | ||||
| Previous manipulation, n (%) | 43 (38.7) | 36 (38.7) | 7 (38.9) | 0.989 |
| Acute (<1 week), n (%) | 26 (23.9) | 22 (23.7) | 4 (25) | 0.907 |
| Pyrexia, n (%) | 103 (92.8) | 85 (91.4) | 18 (100) | 0.350 |
| Dyspnoea or worsening of dyspnoea, n (%) | 62 (55.9) | 52 (55.9) | 10 (55.6) | 0.978 |
| Constitutional syndrome, n (%) | 75 (69.4) | 59 (65.6) | 16 (88.9) | 0.055 |
| Murmur or worsening of murmur, n (%) | 71 (64) | 52 (68.4) | 13 (72.2) | 0.593 |
| Skin manifestations, n (%) | 13 (11.7) | 9 (9.7) | 4 (22.2) | 0.220 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve; HTN: hypertension; DM: diabetes mellitus; AMI: acute myocardial infarction; CVA: cerebrovascular accident; COPD: chronic obstructive pulmonary disease.
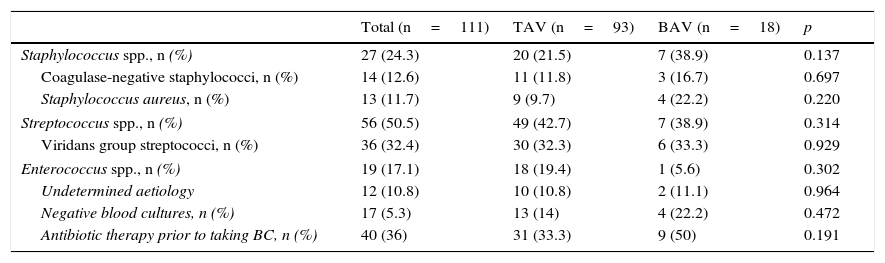
The causative agent of IE was found in the vast majority of patients in both groups, with 10.8% (11.1% vs 10.8%) remaining undefined. There were no differences in the number of cases of IE with negative blood cultures (22.2% vs 14%, p=0.472). In the BAV group, there was a greater tendency towards the aetiology being staphylococcal (38.9% vs 21.5%, p=0.137), particularly Staphylococcus aureus (S. aureus), although without reaching statistical significance. In patients with BAV there was a greater tendency to have taken antibiotics prior to the extraction of blood cultures (50% vs 33.3%, p=0.191) (Table 2).
Microbiological characteristics.
| Total (n=111) | TAV (n=93) | BAV (n=18) | p | |
|---|---|---|---|---|
| Staphylococcus spp., n (%) | 27 (24.3) | 20 (21.5) | 7 (38.9) | 0.137 |
| Coagulase-negative staphylococci, n (%) | 14 (12.6) | 11 (11.8) | 3 (16.7) | 0.697 |
| Staphylococcus aureus, n (%) | 13 (11.7) | 9 (9.7) | 4 (22.2) | 0.220 |
| Streptococcus spp., n (%) | 56 (50.5) | 49 (42.7) | 7 (38.9) | 0.314 |
| Viridans group streptococci, n (%) | 36 (32.4) | 30 (32.3) | 6 (33.3) | 0.929 |
| Enterococcus spp., n (%) | 19 (17.1) | 18 (19.4) | 1 (5.6) | 0.302 |
| Undetermined aetiology | 12 (10.8) | 10 (10.8) | 2 (11.1) | 0.964 |
| Negative blood cultures, n (%) | 17 (5.3) | 13 (14) | 4 (22.2) | 0.472 |
| Antibiotic therapy prior to taking BC, n (%) | 40 (36) | 31 (33.3) | 9 (50) | 0.191 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve; BC: blood cultures.
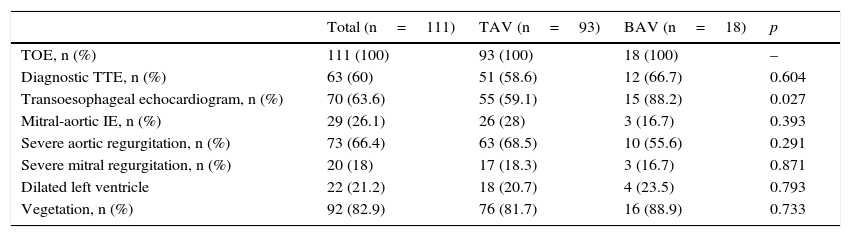
All patients had a transthoracic echocardiogram, with no statistically significant difference in the percentage of patients with echocardiographic evidence of endocarditis (66.7% vs 58.6%, p=0.604). More patients with BAV had a transoesophageal echocardiogram (88.2% vs 59.1%, p=0.027), with findings indicative of IE in 90.1% of cases overall but, once again, there were no differences between the two groups (90.3% vs 89.3%, p=0.640). The percentage of patients with severe aortic regurgitation (AR) was similar in both groups. There were no statistically significant differences in the prevalence of dilated left ventricle (an indicator of chronic AR) among patients with severe AR. Echocardiographic visualisation of vegetation was also similar (88.9% vs 81.7%, p=0.733), as was concomitant mitral valve involvement (16.7% vs 28%, p=0.393) (Table 3).
Echocardiographic characteristics.
| Total (n=111) | TAV (n=93) | BAV (n=18) | p | |
|---|---|---|---|---|
| TOE, n (%) | 111 (100) | 93 (100) | 18 (100) | – |
| Diagnostic TTE, n (%) | 63 (60) | 51 (58.6) | 12 (66.7) | 0.604 |
| Transoesophageal echocardiogram, n (%) | 70 (63.6) | 55 (59.1) | 15 (88.2) | 0.027 |
| Mitral-aortic IE, n (%) | 29 (26.1) | 26 (28) | 3 (16.7) | 0.393 |
| Severe aortic regurgitation, n (%) | 73 (66.4) | 63 (68.5) | 10 (55.6) | 0.291 |
| Severe mitral regurgitation, n (%) | 20 (18) | 17 (18.3) | 3 (16.7) | 0.871 |
| Dilated left ventricle | 22 (21.2) | 18 (20.7) | 4 (23.5) | 0.793 |
| Vegetation, n (%) | 92 (82.9) | 76 (81.7) | 16 (88.9) | 0.733 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve; TTE: transthoracic echocardiogram.
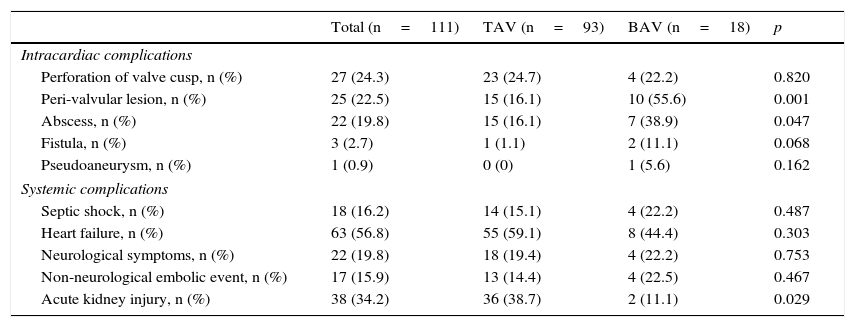
Peri-valvular complications occurred in 25 patients (abscess, fistula or pseudoaneurysm visualised on echocardiogram or during surgery), representing 55.6% of the patients with BAV IE and 16.1% in the TAV group (p=0.001), with abscess being the most common complication found (38.9% vs 16.1%, p=0.047) (Table 4). Using logistic regression analysis, which included other factors possibly associated with the development of peri-valvular complications such as age, Charlson index, S. aureus infection, previous manipulation and duration of symptoms, the presence of BAV was the only predictor of such complications (OR 7.87, 95% CI, 2.38–26.64, p=0.001).
Clinical progress.
| Total (n=111) | TAV (n=93) | BAV (n=18) | p | |
|---|---|---|---|---|
| Intracardiac complications | ||||
| Perforation of valve cusp, n (%) | 27 (24.3) | 23 (24.7) | 4 (22.2) | 0.820 |
| Peri-valvular lesion, n (%) | 25 (22.5) | 15 (16.1) | 10 (55.6) | 0.001 |
| Abscess, n (%) | 22 (19.8) | 15 (16.1) | 7 (38.9) | 0.047 |
| Fistula, n (%) | 3 (2.7) | 1 (1.1) | 2 (11.1) | 0.068 |
| Pseudoaneurysm, n (%) | 1 (0.9) | 0 (0) | 1 (5.6) | 0.162 |
| Systemic complications | ||||
| Septic shock, n (%) | 18 (16.2) | 14 (15.1) | 4 (22.2) | 0.487 |
| Heart failure, n (%) | 63 (56.8) | 55 (59.1) | 8 (44.4) | 0.303 |
| Neurological symptoms, n (%) | 22 (19.8) | 18 (19.4) | 4 (22.2) | 0.753 |
| Non-neurological embolic event, n (%) | 17 (15.9) | 13 (14.4) | 4 (22.5) | 0.467 |
| Acute kidney injury, n (%) | 38 (34.2) | 36 (38.7) | 2 (11.1) | 0.029 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve.
There were no significant differences in the development of septic shock, heart failure, neurological manifestations or embolic events at other sites. However, a lower percentage of patients with BAV developed acute kidney injury (11.1% vs 38.7%) (Tables 1–4).
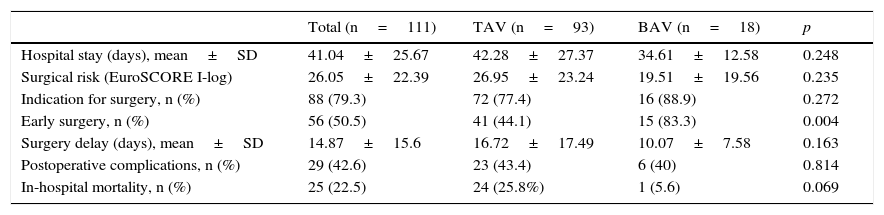
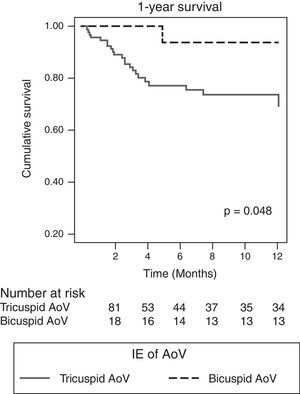
Characteristics of progressThe indication for surgery was similar in both groups (88.9% vs 77.4%, p=0.272), but significantly more patients with BAV IE had surgery during their hospital stay (83.3% vs 44.1%, p=0.004). In-hospital mortality rates were lower in this group, although without reaching statistical significance (5.6 vs 25.8%, p=0.069) (Table 5), and 1-year survival was significantly better (93.8 vs 69.3%, p=0.048) (Fig. 2). After adjusting for parameters possibly associated with survival (age, comorbidity, ejection fraction, presence of BAV, S. aureus infection, finding of severe AR, peri-valvular complications and early surgery), Cox regression analysis revealed that the only predictor of mortality was severe AR (RR 3.73, 95% CI, 1.22–11.36, p=0.021), and early surgery was protective (OR 0.303, 95% CI, 0.097–0.951). Having BAV or peri-valvular complications did not affect risk of death.
Characteristics of progress.
| Total (n=111) | TAV (n=93) | BAV (n=18) | p | |
|---|---|---|---|---|
| Hospital stay (days), mean±SD | 41.04±25.67 | 42.28±27.37 | 34.61±12.58 | 0.248 |
| Surgical risk (EuroSCORE I-log) | 26.05±22.39 | 26.95±23.24 | 19.51±19.56 | 0.235 |
| Indication for surgery, n (%) | 88 (79.3) | 72 (77.4) | 16 (88.9) | 0.272 |
| Early surgery, n (%) | 56 (50.5) | 41 (44.1) | 15 (83.3) | 0.004 |
| Surgery delay (days), mean±SD | 14.87±15.6 | 16.72±17.49 | 10.07±7.58 | 0.163 |
| Postoperative complications, n (%) | 29 (42.6) | 23 (43.4) | 6 (40) | 0.814 |
| In-hospital mortality, n (%) | 25 (22.5) | 24 (25.8%) | 1 (5.6) | 0.069 |
BAV: bicuspid aortic valve; TAV: tricuspid aortic valve.
In this study, 35.6% of the IE cases affected native aortic valves and 18 (16.2%) of those were bicuspid valves (Table 1). That rate is similar to the rates previously published in the literature (Lamas and Eykyn, 2000; Tribouilloy et al., 2010).14,24 The initial diagnosis in our patients with BAV IE was made by transthoracic echocardiogram and confirmed by TOE in about 90% of the cases (Table 3). We should point out that in patients with clear surgical criteria who underwent early surgery (83.3%), the TOE may have been performed intraoperatively. Similar percentages have been described in previous studies with similar numbers of TOEs performed, or even fewer, whether in BAV or TAV.14,25 Although uncommon, BAV IE is a significant problem and our results corroborate the findings of other authors.25 It most often occurs in young males aged 51±19.1 with no comorbidity (mean Charlson index=0.67) (Table 1). We did not find any predominant causal germ, probably because of the small number of cases. However, we did recognise a greater tendency for staphylococcal aetiology (Table 2). We found no differences between BAV and TAV in this study in terms of systemic complications, with both showing similar rates of heart failure, septic shock, neurological events, cardiac emboli to other regions and renal deterioration (Table 4). The important factor in this study of BAV IE was the presence of peri-valvular complications (55.6%), with a statistical difference, despite the small sample size, in relation to TAV (Table 4), with aortic peri-valvular abscess predominating (40%), followed at some distance by fistulae (11%) and pseudoaneurysms (5%). All of the above were reasons for early surgery in these patients (83.3% of the total) and early surgery was required more often than in TAV (Table 5). Despite the poor prognosis associated with the presence of peri-valvular lesions in IE,26,27 the combination of early surgery,28,29 and the young age of these patients and lack of comorbidity means that the in-hospital mortality rate is lower than in TAV: 1 (5.6%) vs 24 (25.8%), p=0.029, with 1-year survival after discharge close to 94%.
These findings, also reported by other authors,14,24,25 lead us to conclude that the development of peri-valvular complications is a constant in BAV IE. It has been postulated that the early onset of haemodynamic changes (a consequence of dysfunctional AoV from an early age) may lead to tissue damage, which is where the germs that cause IE attach themselves. Various reasons have been proposed to explain the higher incidence of peri-valvular complications. The fact that BAV affects young patients with little comorbidity who are unaware of their heart condition may reduce the suspicion index for this disease. This delays both diagnosis (especially in cases with negative blood culture, generally after previous antibiotic therapy for other possible foci) and the appropriate treatment, leading to greater local spread of the cardiac lesions. At the same time, IE of staphylococcal aetiology, particularly S. aureus thanks to its aggressive nature, contributes to greater and faster tissue destruction.
With regard to limitations of our study, the first and main one is the small number of patients included with BAV, primarily resulting from a probable underestimation of the prevalence of BAV, as techniques which allow the maximum morphological definition of the valve (such as a transoesophageal echocardiogram [TOE]) were not used in all patients, and nor did all patients with AoV IE have surgical intervention. The second limitation is the long period of study. It is our opinion that both limitations are common to the work by other authors already mentioned in the text.
ConclusionsIn our population, patients with BAV IE are younger and have less comorbidity than carriers of IE of tricuspid AoV. However, there is a higher percentage of peri-valvular complications, requiring early surgery in most cases. In spite of this, mortality rates in-hospital or in the month following discharge are lower, and survival at one year is significantly better.
Conflicts of interestThere are no conflicts of interest related to the publication of this article.
Please cite this article as: Becerra-Muñoz VM, Ruíz-Morales J, Rodríguez-Bailón I, Sánchez-Espín G, López-Garrido MA, Robledo-Carmona J, et al. Endocarditis infecciosa sobre válvula aórtica bicúspide: características clínicas, complicaciones y pronóstico. Enferm Infecc Microbiol Clin. 2017;35:645–650.