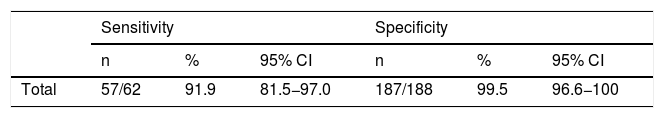
Measles, a notifiable disease for which vaccination is available,1 is the target of a plan for elimination in Spain.2 In Spain, measles is no longer a paediatric disease, giving rise to outbreaks, often of imported origin, that increasingly affect adults.3 Despite the disruption of continued endemic transmission, the incidence of the infection in Europe has increased in recent years, associated with decreases in vaccine coverage.4 Clinical suspicion, based on the presence of maculopapular exanthema accompanied by cough, rhinitis and or conjunctivitis, requires laboratory confirmation.1,2 Serological diagnosis by specific IgM detection is difficult in previously immunised subjects, who may have false negative results, and its use is supplemented with molecular amplification techniques by reverse transcription-polymerase chain reaction (RT-PCR).5 The Laboratorio de Referencia e Investigación en Enfermedades Víricas Inmunoprevenibles del Centro Nacional de Microbiología [Reference and Research Laboratory for Vaccine-Preventable Viral Diseases of the Spanish National Microbiology Centre] (LRIEVI-CNM) has a conventional (non real-time) multiple RT-PCR technique that enables simultaneous detection of the measles, rubella and parvovirus B19 viruses.6,7 Although some real-time RT-PCR techniques for measles are available on the market,8 there is little information on the usefulness of new kits. The objective of this study was to evaluate the performance of a real-time RT-PCR kit (Measles Virus Real Time RT-PCR Kit, Shanghai ZJ Bio-Tech Co.) for the diagnosis of measles virus infection. A total of 250 pharyngeal exudate samples, collected from patients with suspected measles and received at the Laboratorio Regional de Salud Pública [Regional Public Health Laboratory] (LRSP) in the Autonomous Community of Madrid between 2012 and 2019, were processed. All samples were tested using the Measles Virus Real Time RT-PCR Kit and the reference method for RT-PCR of the LRSP/CNM.6,7 Separate extractions of nucleic acids were performed for use in the RT-PCR technique evaluated and in the reference technique. For the extraction in the Measles Virus Real Time RT-PCR Kit technique, the Qiagen EZ1 instrument was used in combination with EZ1 kits (Qiagen GmbH). For each sample, the initial volume was 200 μl, and, after completing the extraction process, 5 μl of RNA were added to 20 μl of the master mix (for a final volume for the RT-PCR reaction of 25 μl). The extraction for the multiple conventional RT-PCR reference technique used the QIAsymphony DSP Virus/Pathogen Midi Kit (Qiagen GmbH), a sample volume of 400μl and elution volume of 40 μl, 72 samples tested positive using the reference method, and 57 of them also tested positive using the technique evaluated. Among the 188 samples that tested negative using the reference procedure, 187 (10 of which were positive for parvovirus B19 and two of which were positive for rubella) also tested negative using the Measles Virus Real Time RT-PCR Kit. Table 1 shows the sensitivity and specificity results for the technique studied, expressed in terms of percentages and corresponding 95% confidence intervals (95% CI). Despite being included in the systematic vaccination schedule, measles causes sporadic outbreaks.9 In the context of the Plan Nacional de Eliminación del Sarampión [Spanish National Plan for the Elimination of Measles], it has been deemed necessary to quickly identify all suspected cases with high specificity criteria.2 One limitation of this study lies in the fact that the samples were not processed using the technique evaluated and the reference technique based on the same elution. This may have led to differences that could not be ascribed to RT-PCR and might have actually resulted from extraction failures. The real-time RT-PCR technique assessed in this study is simple, and it has a high specificity and acceptable sensitivity. The number of false negatives for this commercial technique may have repercussions for its clinical application, especially given the highly contagious nature of measles. However, it may be suitable for confirming suspected cases quickly, provided that negative cases are investigated using a definitive method with higher sensitivity, such as the LRIEVI-CNM technique used in this study.
The authors declare that they have no conflicts of interest.
Please cite this article as: Sanz JC, Fernández-García A, Echevarría JE, de Ory F. Valoración de un kit comercializado de RT-PCR para el diagnóstico de la infección por el virus del sarampión. Enferm Infecc Microbiol Clin. 2021;39:155–156.