Intensive care unit (ICU)-acquired weakness is developed by 40%–46% of patients admitted to ICU. Different studies have shown that Early Mobilisation (EM) is safe, feasible, cost-effective and improves patient outcomes in the short and long term.
ObjectiveTo design an EM algorithm for the critical patient in general and to list recommendations for EM in specific subpopulations of the critical patient most at risk for mobilisation: neurocritical, traumatic, undergoing continuous renal replacement therapy (CRRT) and with ventricular assist devices (VAD) or extracorporeal membrane oxygenation (ECMO).
MethodologyReview undertaken in the Medline, CINAHL, Cochrane and PEDro databases of studies published in the last 10 years, providing EM protocols/interventions.
Results30 articles were included. Of these, 21 were on guiding EM in critical patients in general, 7 in neurocritical and/or traumatic patients, 1 on patients undergoing CRRT and 1 on patients with ECMO and/or VAD. Two figures were designed: one for decision-making, taking the ABCDEF bundle into account and the other with the safety criteria and mobility objective for each.
ConclusionsThe EM algorithms provided can promote early mobilisation (between the 1st and 5th day from admission to ICU), along with aspects to consider before mobilisation and safety criteria for discontinuing it.
La debilidad adquirida en la unidad de cuidados intensivos (DAU) es desarrollada por el 40–46% de los pacientes ingresados en UCI. Diferentes estudios han mostrado que la movilización temprana (MT) es segura, factible, costo-efectiva y mejora los resultados del paciente a corto y largo plazo.
ObjetivoDiseñar un algoritmo de MT para el paciente crítico en general y enumerar unas recomendaciones para la MT en subpoblaciones específicas de paciente crítico con más riesgo para la movilización: neurocrítico, traumático, sometido a terapias continuas de depuración renal (TCDR) y con dispositivos de asistencia ventricular (DAV) o membrana de oxigenación extracorpórea (ECMO).
MetodologíaRevisión en las bases de datos Medline, CINAHL, Cochrane y PEDro de estudios publicados en los últimos 10 años, que aporten protocolos/intervenciones de MT.
ResultadosSe incluyeron 30 artículos. De ellos, 21 eran para guiar la MT en el paciente crítico en general, 7 en pacientes neurocríticos y/o traumáticos, uno en pacientes portadores de TCDR y uno en pacientes portadores de ECMO y/o DVA. Se diseñan 2 figuras: una para la toma de decisiones teniendo en cuenta el bundle ABCDEF y la otra con los criterios de seguridad y objetivo de movilidad para cada uno.
ConclusionesLos algoritmos de MT aportados pueden promover la movilización precoz (entre el 1.er y 5.o día de ingreso en UCI), junto a aspectos a tener en cuenta antes de la movilización y criterios de seguridad para suspenderla.
Intensive care unit-acquired weakness (ICU-AW) is common amongst survivors of critical illnesses. This syndrome consists in atrophy and/or loss of muscle mass with consequent myopathy, polyneuropathy or both at the same time, with no other explanatory aetiology than the critical pathology itself, and begins 24 h after admission to the ICU, and then progresses.1 Among the risk factors the following stand out: sepsis, multi organ failure, mechanical ventilation (MV), immobilisation and hyperglycaemia.2,3 Several studies show that ICU-AW occurs in 40%–46% of patients admitted to the ICU.4,5 The development of ICU-AW is associated with poorer short and long-term outcomes, including difficulty or failure to wean, a longer stay in the ICU and hospital, an increase in mortality and a poorer functional status with persistent disability in daily life activities, which may even remain 5 years after hospitalization. This condition also increases healthcare costs and leads to the deterioration of patients’ quality of life.6,7
For prevention and treatment, numerous research studies have described the benefit of the early mobilization programmes in patients admitted to ICUs.8–16
Although consensus is lacking in a definition of early mobilisation (EM),17 this physical activity is considered to be of intense and early application, between the 2nd and 5th day of admission to the ICU.18,19 Half of the studies which have defined it consider this to be so.20 These studies showed that EM is safe, feasible, cost-effective and improves short and long-term patient outcomes.10,16,21–25 Furthermore, the most recent clinical guide to approach EM26 indicates that it strengthens the muscles on discharge from the ICU and reduces MV days, and recommends the implementation of EM into the package or set of measures, known as the ABCDEF bundle (A and B: awake and breathing; C: choice of sedatives and analgesics; D delirium prevention; E: early mobilisation; F: family empowerment). This constitutes an evidence-based guide for establishing algorithms of sedation, prevention-management of delirium and EM.26
Justification of the need to create an early mobilisation guide (EM)Despite the awareness of the damaging effects immobility has and the extensive benefits of EM, as already commented upon, it is not a practice that has been integrated into daily care. Only 14% of the 86 ICUs interviewed in Spain had implemented EM protocols or algorithms.27 This may be conditioned by the existence of different barriers which include lack of knowledge by the staff and the variability of care, fear of falls, pain during mobilization, physiological instability of the patient, over-sedation, lack of human and technical resources and lack of time, insufficient collaboration between the interprofessional team and an absence of specific protocols.23,28–32
In our environment there is also very little presence of physiotherapists in the ICUs27,33 and on a geographical level, training and competences of physiotherapists are highly variable.33–37
The European Respiratory Society and the European Society of Intensive Care Medicine Task Force on Physiotherapy for Critically Ill Patients recommend developing clinical physiotherapy guidelines, identifying patient characteristics of those susceptible to this treatment and increasing awareness among professionals of the damaging effects of immobility and the benefits of EM.14
In keeping with the above, the general objective of this study was to create an EM guideline to be implemented in daily ICU practice, for the purpose of increasing patients’ physical activity: sitting up and getting out of bed.
Specific objectives were to:
- 1
Propose validated tools to measure the degree of mobilisation achieved by the patient and to assess their functional capacity.
- 2
Identify the patients at greatest risk of ICU-AW.
- 3
Design an EM algorithm for the critical patient in general.
- 4
List several recommendations for EM in specific sub-populations of the critical patient most at risk regarding mobilization: neurocritical, traumatic, subjected to continuous renal replacement therapies (CRRT) and with ventricular assist devices (VAD) or extracorporeal oxygenation membranes (ECMO).
Professionals who care for critical patients: nurses, physicians (intensive care, anaesthetists, rehabilitators) physiotherapists and occupational therapists.
Study populationPatients in multi-purpose ICUs, doctors, heart and/or coronary surgeons, surgical, trauma, postsurgical resuscitation and semicritical care units.
Patients undergoing rehabilitation who were hospitalised in long-stay units or in hospital wards were not considered in this guide, even though they had been in the ICU.
Resources usedA bibliographic review was undertaken which was completed on 1st December 2019. The following criteria were considered:
- ‐
inclusion: critically ill patient, admitted into the acute ICU, studies which provided an algorithm/guide/protocol/EM interventions.
- ‐
exclusion: studies focusing only on rehabilitation therapies such as the use of electrostimulation or mobilisation interventions in end-of-life patients.
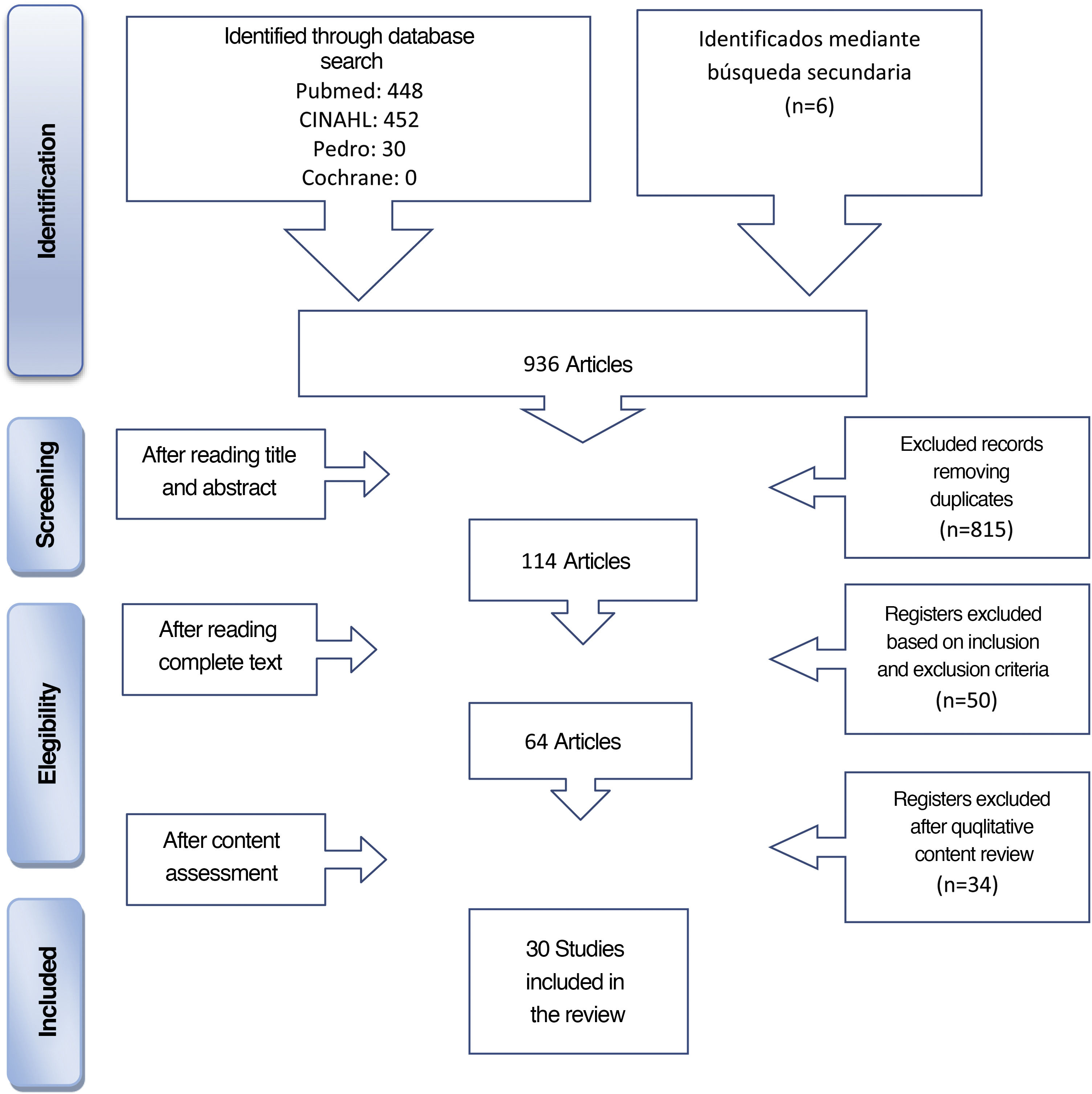
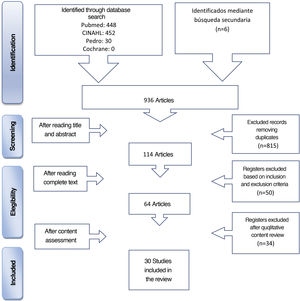
The databases consulted were Medline (vía PubMed), CINAHL, PEDro and the Cochrane Library. The selection process of articles is contained in the Flow diagram (Fig. 1).
The limits used were articles published in the last 10 years (2009–2019), language (English and Spanish), selected for adults aged = 19 or above.
The following MeSH terms were used for the search of related scientific literature in the databases of Medline (PubMed), CINAHL and the Cochrane Library: “rehabilitation, “exercise therapy”, “early ambulation”, “intensive care units”, “critical care”. In the PEDro databases the terms were as follows: “rehabilitation”, “exercise therapy”, “early ambulation”, “early mobilizationͭ”, “intensive care units”, “critical care”. In both cases these were combined with the Booleans AND and OR.
In addition, an inverse search was made from the bibliographic references of selected studies, together with other unidentified sources from the review. These comprise the articles cited as secondary review in Fig. 1.
Analysis of resultsThe results obtained were analysed independently by 5 of the authors of this review (MRT, ERM, BPP, JDM y GZE), bearing in mind the study objectives and the inclusion and exclusion criteria.
A quantitative review of the algorithm/protocols/EM interventions or early EM was also undertaken with the following criteria:
- ‐
Variables recommended for guiding the mobilisation level: consciousness and physical function.
- ‐
Use of mobilization support apparatus.
- ‐
Times recommended for each physiotherapy activity.
Thirty articles were included which provided an algorithm, protocol or EM intervention, which provided a response to the objective of this study. Of these, 21 were on guiding EM in the critical care patient in general, 7 in the neurocritical patient and/or trauma patients, one in patients on continuous renal replacement therapy (CRRT) and one in ECMO and/or ventricular assist device (VAD) patients (Appendix A Table 1 of Supplementary material).
Levels of evidence of the selected studiesOf the 30 studies included, as shown in Appendix A Table 1 of the Supplementary material, 10 held evidence levels 1A and 1B (randomised clinical trials), 17 evidence levels 2B, 2C and 2D (cohort studies, of cases and controls or pre-post studies), 2 level 3D (case reports) and one level 4D (expert opinion).
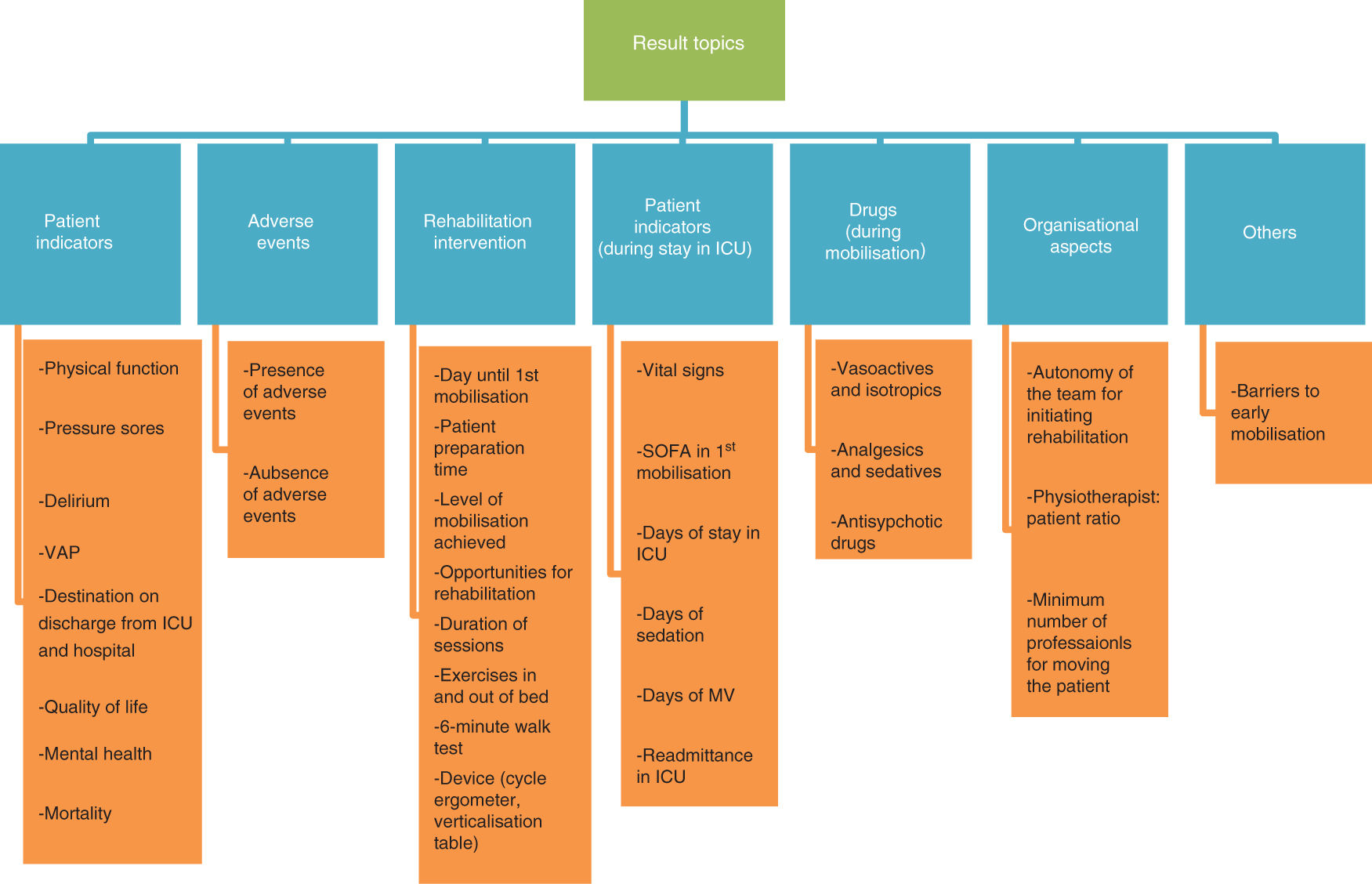
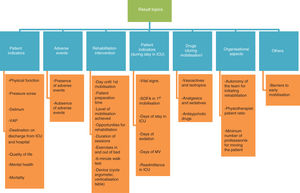
Variability of resultsThe heterogeneity of the results found in the current literature impeded study comparison. Fig. 2 shows this variability, grouped into 7 different topics.
Also, despite the previously described advantages of EM, according to available evidence, it does not involve changes to quality of life, mortality in the hospital or changes in physical health. It cannot be assessed whether there are effects in cognitive function, mental health or the capacity to return to work, due to insufficiency of data.26 The latest Cochrane review provides similar results: lack of evidence to determine whether EM improves daily life activities, muscle strength or quality of life.38
Validated tools for measuring the degree of mobilisation achieved by the patient and assessment of their functional capacityThe tools are classified into those that measure muscle mass (anthropometry, bioimpedance, ultrasonography), muscle weakness (manual testing with Medical Research Council-Sum score [MRC-SS] or dynamometer) and physical function with this dimension being the one with the most developed tools, but few which are suitably validated. They best ones are those with psychometric qualities which are the Chelsea critical care physiotherapy (CPAx), Physical Function in Intensive Care Test (PFIT) and the ICU Mobility Scale (IMS).39
On the one hand, to assess muscle weakness, both the manual test of the muscle and the dynamometry require the patient’s cooperation. The most used scale for manual testing is the MRC-SS. This assesses muscle strength from 0 (no muscle contraction) to 5 (maximum contraction). Three muscle groups are examined in each of the upper and lower limbs, and the total score is over 60. Accessible evaluation protocol can be consulted in https://seeiuc.org/estudio-movipre/, adapted from Hermans et al.40
On the other, with regard to physical function, the IMS was created to replace the 6-min walk test,41,42 not applicable in the critical patient and it is the only mobility scale designed to standardize the language of nurses and physiotherapists when they describe patient mobilization during ICU stay.43,44
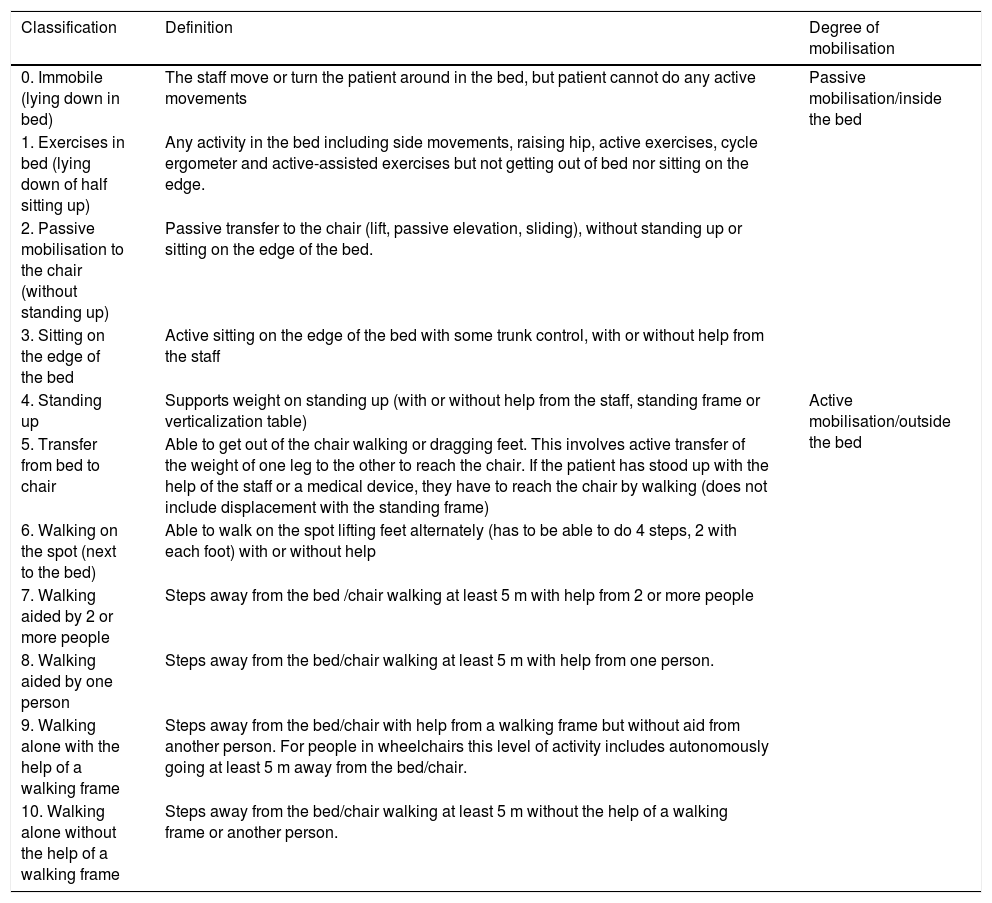
One limitation of many studies which have assessed the effectiveness of early mobilisation in the ICU is that of not having used a validated mobility scale to define the different degrees of activity the patients achieve. The IMS was therefore validated into the Spanish cultural context, with recommendations from experts for the tool to have an equivalent semantic, and conceptual level of technical content and criteria in the different languages45 (see Table 1).
Mobility scale (IMS-Spain).
| Classification | Definition | Degree of mobilisation |
|---|---|---|
| 0. Immobile (lying down in bed) | The staff move or turn the patient around in the bed, but patient cannot do any active movements | Passive mobilisation/inside the bed |
| 1. Exercises in bed (lying down of half sitting up) | Any activity in the bed including side movements, raising hip, active exercises, cycle ergometer and active-assisted exercises but not getting out of bed nor sitting on the edge. | |
| 2. Passive mobilisation to the chair (without standing up) | Passive transfer to the chair (lift, passive elevation, sliding), without standing up or sitting on the edge of the bed. | |
| 3. Sitting on the edge of the bed | Active sitting on the edge of the bed with some trunk control, with or without help from the staff | |
| 4. Standing up | Supports weight on standing up (with or without help from the staff, standing frame or verticalization table) | Active mobilisation/outside the bed |
| 5. Transfer from bed to chair | Able to get out of the chair walking or dragging feet. This involves active transfer of the weight of one leg to the other to reach the chair. If the patient has stood up with the help of the staff or a medical device, they have to reach the chair by walking (does not include displacement with the standing frame) | |
| 6. Walking on the spot (next to the bed) | Able to walk on the spot lifting feet alternately (has to be able to do 4 steps, 2 with each foot) with or without help | |
| 7. Walking aided by 2 or more people | Steps away from the bed /chair walking at least 5 m with help from 2 or more people | |
| 8. Walking aided by one person | Steps away from the bed/chair walking at least 5 m with help from one person. | |
| 9. Walking alone with the help of a walking frame | Steps away from the bed/chair with help from a walking frame but without aid from another person. For people in wheelchairs this level of activity includes autonomously going at least 5 m away from the bed/chair. | |
| 10. Walking alone without the help of a walking frame | Steps away from the bed/chair walking at least 5 m without the help of a walking frame or another person. |
As commented upon in the introduction, since 2014 different ICU-AW-associated risk factors have been identified: These include age,19,46 greater degree of dependence in daily life activities,1,19 hyperglycaemia3,46 and administration of corticoids,3,46 but none of the studies were conducted in Spain, and it is therefore unknown whether specific ICU characteristics exist that may impact the incidence of these factors, or add several new ones, bearing in mind the Spanish organizational and cultural environment.
The authors of this paper conducted a national multicentre study (MoviPre, Movilización Precoz)47 in 80 ICUs in Spain during the months of March to July 2017. These included 642 patients (62.1% medical patients, 32.4% surgical patients and 5.5% trauma patients). Using logistic regression analysis (for the period between days 3 and 5 of hospitalization, when the EM should be implemented) they identified the following risk factors of developing ICU-AW: older age (OR 1.1 95% CI [1.00–1.03]) and more days with continuous renal replacement therapy (CRRT) (OR 1.01 95% CI [1.00–1.02]). In contrast, the following protect against the development of ICU-AW : being male (OR .58 95% CI [.38–.89]), a higher score on the Barthel scale (OR .97 95% CI [.95–.99]), more days collaborating for the evaluation of the MRC (OR .98 95% CI [.97–.99]) or developing hyperactive delirium (OR .98 95% CI [.97–.99]) and more days with active mobilization (IMS ≥ 4) (OR .98 95% CI [.97–.99]).
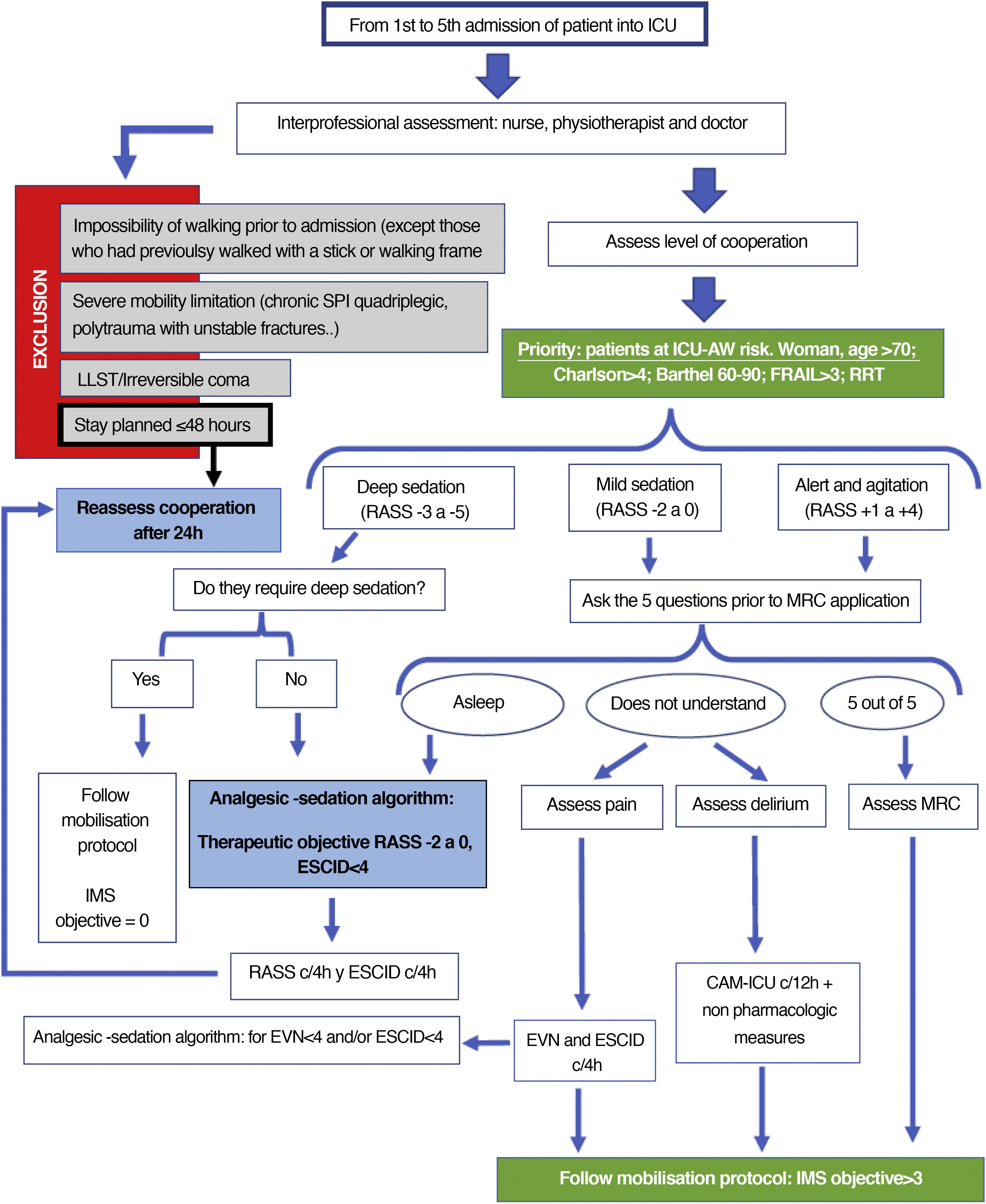
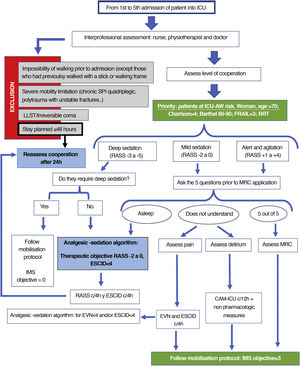
Aspects to bear in mind prior to mobilisation (adapted from Nydahl48)- ‐
Having a portable ventilator and monitor, aspiration device, oxygen, manual resuscitator (if IMS ≥ 7).
- ‐
Anticipate: think which safety risk may appear during the mobilisation of this patient and what strategies should be taken in to account.
- ‐
Consider the asepsis regulation, particularly disconnection of invasive airways.
- ‐
It is recommended to have an intensive care doctor nearby.
- ‐
Change the length of the infusion tubes and systems, in keeping with the level of mobilisation pursued.
- ‐
Consider tube and infusion system attachments.
- ‐
Assess whether pressure in the airways is not too high for leaving a margin in case it may increase during mobilisation.
- ‐
Have a chair available nearby for the patient to sit on if they need to (wheelchair).
- ‐
Consider the employment safety risks for professionals and the appropriate strategies to mitigate them.
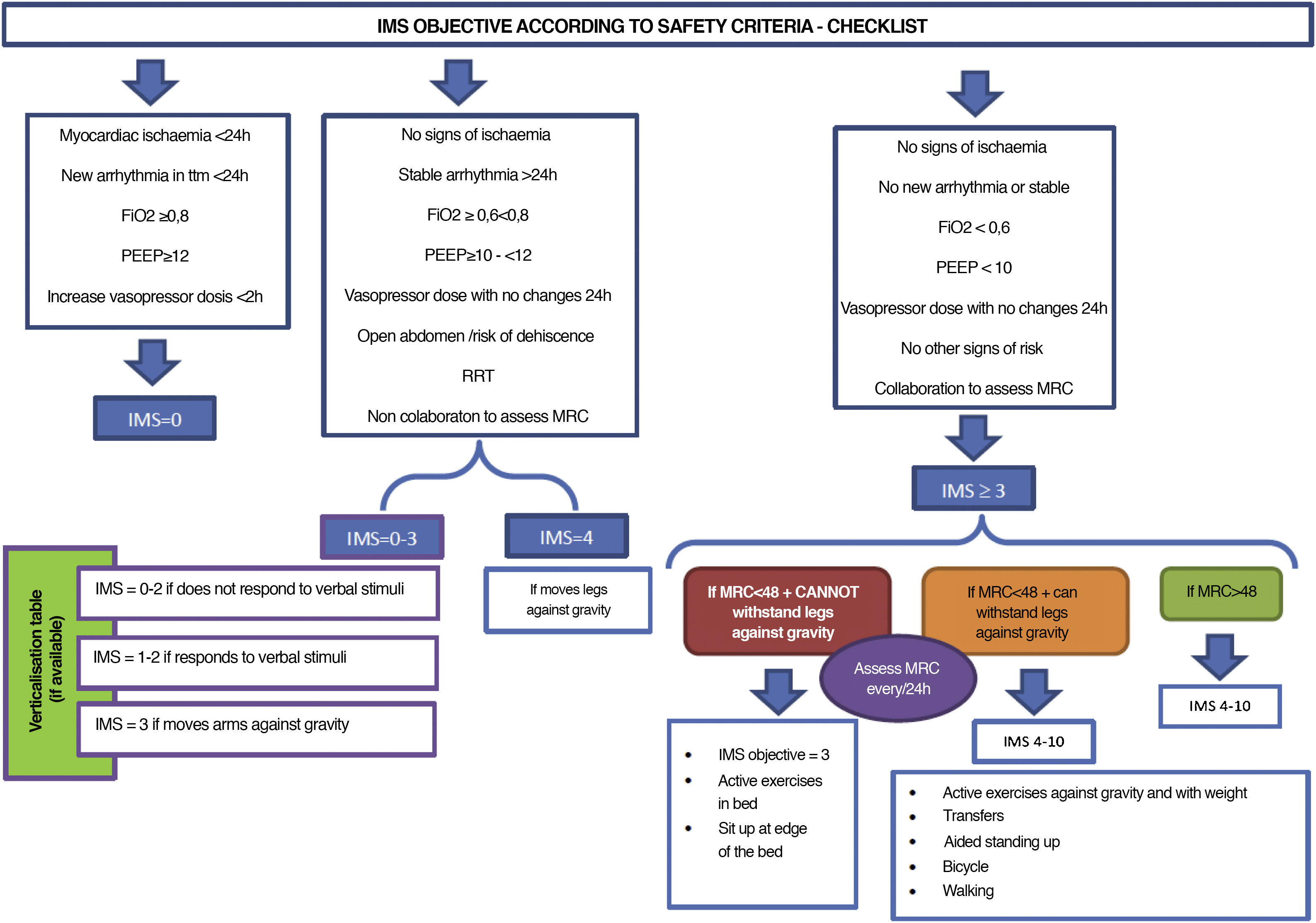
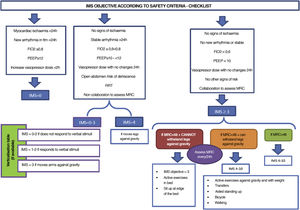
Fig. 3 (decision making) and Fig. 4 (IMS objective in keeping with safety checklist).
Safety criterion for discontinuation of early mobilisation (adapted from Nydahl48)- ‐
Change in systolic blood pressure (SBP) > 20%, compared with baseline at rest.
- ‐
Heart rate (HR) > 200-(age in years).
- ‐
Drop in oxygen saturation 5%, compared with baseline at rest.
- ‐
Breathing effort according to the Borg scale ≥ 7 (0 = very easy, 10 = maximal exertion). First, try to increase the breathing pressure in the ventilator by 4 mbar and reassess. If there is no improvement, stop mobilisation.
- ‐
Physical training exertion according to the Borg scale ≥ 7 (0 = very easy, 10 = maximal exertion). First try a short rest (e.g., sit down on a chair for one minute) and reassess: if there is no improvement stop mobilisation.
It is recommended that if therapy is intermittent haemodialysis mobilisation should be made before or after it. If therapy is continuous renal replacement therapy (CRRT) the patient can only be mobile inside the room (IMS maximum of 6), due to the difficulty in lengthening treatment (arterial and venous) and feed lines of the system.49
It is also recommended not to go beyond 90° of hip flexion in the presence of femoral catheters.50
For neurocritical and/or trauma patientsIMS > 3 is contraindicated in the presence of unstable fractures and IMS < 3 is recommended if there is rachis instability.51
Only moving neurocritical patients who present with steady intracranial pressure of < 20 mmHg for patient stimulation52,53 or a motor response of > 428 on the Glasgow coma scale.
Exclusion criteria for mobilisation of the neurocritical patient: not tolerating the pinching of the ventricular draining for a minimum of 30 min, sustained intracranial pressure over 20 mmHg or neurological fluctuations during the examination prior to mobilisation.53
The following activities are proposed depending on the restriction criterion of raising the bed headboard:28
For neurocritical care patients with subarachnoid haemorrhageOlkowsky et al.54 proposed the following inclusion criteria for early mobilisation of these patients:
- ‐
Aneurism resolved with or without any underlying identified aneurism.
- ‐
Lindegaard ratio ≤ 3.0 or mean speed of blood flow in middle cerebral artery ≤ 120 cm/s.
- ‐
Mean blood pressure (MBP) between 80 and 110 mmHg.
- ‐
Heart rate (HR) between 40 and 130 bpm.
- ‐
Respiratory rate (RR) ≤ 40 bpm.
- ‐
Saturation of O2 ≥ 88%.
- ‐
Intracranial pressure (ICP) ≤ 15 mmHg.
- ‐
No proof of convulsions.
- ‐
Has stable neurological assessment.
- ‐
Eye opening on verbal stimulus.
- ‐
Has the ability to move a limb when instructed to do so.
The safety checklist criteria were changed prior to mobilisation, in accordance with the following inclusion criteria:55
- ‐
PaO2/FiO2 ≥ 200.
- ‐
PEEP ≤ 7 cmH2O.
- ‐
Saturation of O2 < 90%.
- ‐
2 or fewer vasoactive agents or dose reduction.
- ‐
Heart rate (HR) between 60 and 120 bpm.
- ‐
Middle artery pressure (MAP) between 55 and 120 mmHg.
- ‐
Systolic blood pressure (SBP) between 90 and 180 mmHg.
- ‐
Respiratory rate (RR) between 10 and 30 bpm.
The authors have no conflict of interests to declare.
Please cite this article as: Raurell-Torredà M, Regaira-Martínez E, Planas-Pascual B, Ferrer-Roca R, Martí JD, Blazquez-Martínez E, et al. Algoritmo de movilización temprana para el paciente crítico. Recomendaciones de expertos. Enferm Intensiva. 2021;32:153–163.
This paper was endorsed by the following scientific societies: Sociedad Española de Enfermería Intensiva y Unidades Coronarias (SEEIUC), Sociedad Española de Medicina Crítica Intensiva y Unidades Coronarias (SEMICYUC) and Sociedad Española de Neumología y Cirugía Torácica (SEPAR).