To determine the incidence of moisture-associated skin damage (MASD) in the nappy area, identify predisposing factors and know the preventive measures and nursing records.
MethodDescriptive longitudinal study (June 2014–April 2015) in a general ICU. Patients whose stay >48h and without skin lesions were included. The skin was assessed daily until the appearance of MASD, discharge or a maximum of 14 days. Demographics, stay, MASD type, incontinence, number and consistency of stools, obesity, Braden scale and prevention were recorded.
Results145 patients (66.2% male) were studied, median age was 69 (P25=56.5, P75=76) and median length of stay was five days (P25=3, P75=11.25), 29.9% were obese. Incontinence-associated dermatitis (IAD) was detected in 26.2% and intertriginous dermatitis (ITD) in 15.9%. MASD was recorded in 23.8%. The variables causing IAD to develop were faecal incontinence, number of stools, liquid stools, and stay. Those for ITD were obesity and score on the Braden scale. Multivariate analysis selected faecal incontinence (OR=5.4, CI 95%: 1.1–26) and the number of stools (OR=1.1, CI 95%: 1.0–1.2) as independent variables for developing IAD and obesity (OR=2.8, CI 95%: 1.0–8.2) and Braden (OR=0.8, CI 95%: 0.7–1.0) for developing ITD. Prevention to 23.8% of obese and 42.9% of incontinent was performed.
ConclusionsThere is a high incidence in MASD. Faecal incontinence and higher number of stools are the risk factors for developing IAD. Obesity and a lower score on the Braden scale may affect susceptibility to ITD. Recording of MASD and its prevention in patients at risk is insufficient.
Determinar la incidencia de las lesiones cutáneas asociadas a la humedad (LESCAH) en el área del pañal, identificar los factores predisponentes y conocer las medidas preventivas y registros realizados.
MetodologíaEstudio descriptivo longitudinal (junio de 2014-abril de 2015) en una UCI polivalente. Se incluyeron pacientes con estancia >48 h y sin lesiones cutáneas. Se valoró diariamente la piel hasta la aparición de LESCAH, alta o un máximo de 14 días. Se registraron datos demográficos, estancia, tipo de LESCAH, incontinencia, consistencia y número de deposiciones, obesidad, escala Braden y prevención.
ResultadosSe estudiaron 145 pacientes (66,2% hombres), la mediana de edad fue 69 (P25=56,5-P75=76) años y la estancia de 5(P25=3-P75=11,25) días, el 29,9% presentó obesidad. Se detectó un 26,2% de dermatitis asociada a la incontinencia (DAI) y un 15,9% dermatitis intertriginosa (DI). Se registró el 23,8% de las LESCAH. Las variables relacionadas con la DAI fueron la incontinencia fecal, número de deposiciones, heces líquidas y estancia. Para la DI fueron la obesidad y la puntuación en la escala Braden. El análisis multivariable seleccionó la incontinencia fecal (OR=5,4; IC 95%:1,1–26) y el número de deposiciones (OR=1,1; IC:1,0-1,2) como variables independientes para desarrollar DAI y la obesidad (OR=2,8; IC95%:1,0-8,2) y escala Braden (OR=0,8; IC95%:0,7-1,0) para desarrollar DI. Se realizó prevención al 23,8% de los obesos y al 42,9% de los incontinentes.
ConclusionesExiste una elevada incidencia en LESCAH. Tener incontinencia fecal y mayor número de deposiciones son factores de riesgo para desarrollar DAI. La obesidad y una puntuación menor en la escala Braden predisponen a sufrir DI. El registro de las LESCAH y la prevención en pacientes de riesgo es insuficiente.
Moisture-Associated Skin Damage is considered to be lesions related to dependency. Critically ill patients have a higher risk of developing these. However, this has barely been studied in this population group.
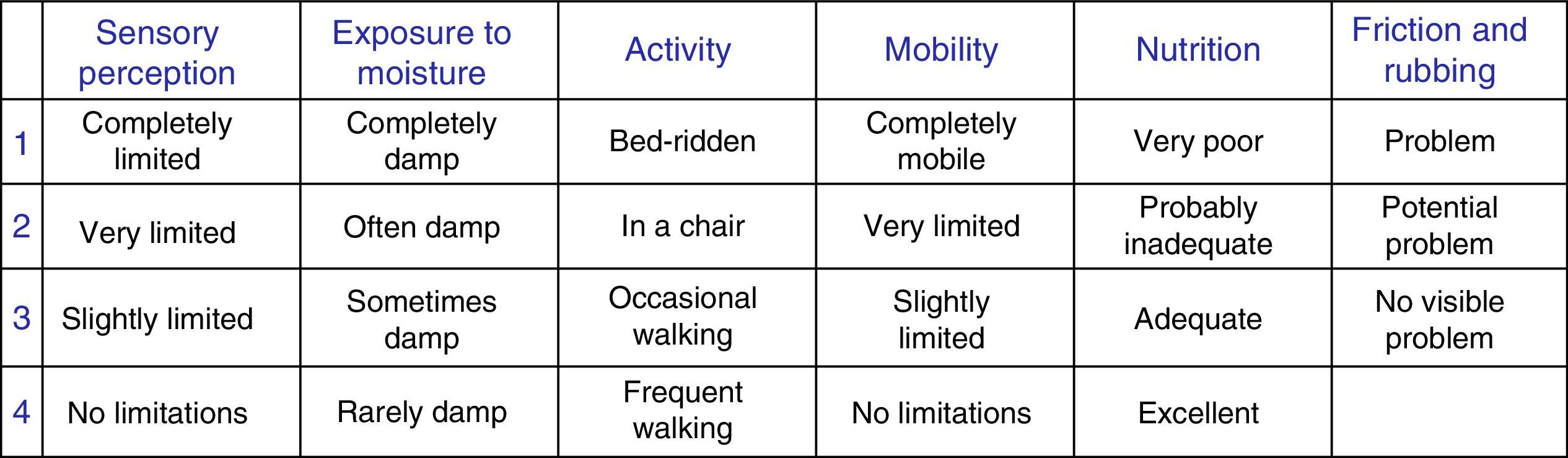
Moisture-Associated Skin Damage is much more common in patients admitted to the ICU. Critically ill patients are vulnerable to lesions as a result of incontinence, but there are other causes too, such as sweating, to which they are also exposed. Moisture is a factor that is overlooked by the people in charge of caring for patients. The Braden scale allows us to assess the exposure of the skin to moisture in general terms.
Implications of the study?It is necessary to raise awareness among professionals about Moisture-Associated Skin Damage, especially intertriginous dermatitis. We need scales to assess the risk of exposure to the different sources of moisture, as well as to examine how the bedsore risk scales work to set the predictive value for this type of lesions.
The skin is the organism's first barrier and there are various factors that can damage it. Among these we find factors that are intrinsic to old age, patients’ medical history, nutritional status or incontinence, inter alia, and external factors such as prolonged immobility, pressure, friction and moisture.1 Patients admitted to Intensive Care Units (ICU) experience most of these risk factors.
El Grupo Nacional para el Estudio y Asesoramiento en Úlceras por Presión y Heridas Crónicas (‘The Spanish National Group for the Study and Consultancy on Bedsores and Chronic Wounds’, GNEAUPP in its initials in Spanish) published its Technical Document No. II regarding lesions connected with dependency in 2014.2 This document describes lesions of various aetiologies, such as bedsores, rubbing or friction and Moisture Associated Skin Damage (MASD). The latter were not long ago described and differentiated from bedsores,3 and they are described as “a lesion on the skin (which does not usually affect the adjacent tissues) which appears as an inflammation (erythema) and/or the erosion of the skin, caused by prolonged exposure (continuous or almost continuous) to various sources of moisture which can cause skin irritation (for example: urine, faeces, exudate from wounds, stoma or fistula effluents, sweat, saliva or mucus).”2
Based on the work of the expert team led by Gray,4 in which the evaluated strategies for assessing, preventing and treating MASD, Torra i Bou et al.5 described six forms of MASD: Incontinence Associated Dermatitis (IAD), intertriginous Dermatitis (ID), Periwound Moisture-Associated Dermatitis, Peristomal Dermatitis, Exudate-Associated Cutaneous Dermatitis (EACD) and dermatitis due to saliva or mucus.
IAD is described as damage caused to the skin due to exposure to urine or faeces.6 It normally develops in the area around the genitals or the area around the anus, but it can spread to the inguinal region, the gluteus and the legs7 the incidence rate is between 3.4% and 50%8 with higher rates being seen in units for the critically ill.
Furthermore, ID is defined as “a lesions caused by moisture on two facing surfaces on the skin that come into contact and tends to appear in the inframammary fold, the armpit or the inguinal region”.7 With these lesions, superinfection from bacteria or fungi is frequent and tends to be more common in obese patients.7
MASD is often found in the nappy area and may be wrongly diagnosed as Grade I or II bedsores and vice versa.9 This confusion may be due to the similarity of the lesions or the fact that 37.6% of bedsores are found in this region (genitals, gluteus and sacrum), as shown by the latest Spanish national study on the prevalence of bedsores.10 Furthermore, in the ICU where the investigation was done, a study was conducted among nurses which indicated this confusion between bedsores and MASD.11 Given the impact that that this has on preventative measures and the incorrect use of resources, it was decided to carry out this study with the aim of determining the incidence rate of MASD in the nappy area, identifying the preventative measures employed in the ICU against such lesions and the documentation in the nursing registers.
MethodologyLongitudinal descriptive study from June 2014 to April 2015 in a polyvalent ICU with 12 beds in a level 3 university hospital.
This included patients over the age of 18, staying for over 48h. Patients who already presented lesions of any kind in the nappy area (which could have made it difficult to assess the area), those declared brain dead, or those who could not have their nappy area assessed daily due clinical orders were excluded.
During the time of the study, there was no protocol in place for the prevention of MASD, with hygiene being carried out on the nappy area with water and soaped sponges, alongside the use of 25% zinc oxide cream as a barrier product as a preventative measure.
From the time the patient was hospitalised, the skin in the nappy area was assessed daily. This assessment was stopped if MASD appeared (erythema/skin erosion), or if there was no lesions, when the patient was discharged from the unit, death or after a maximum of 14 days.
Demographic data (age and sex) diagnosis upon hospitalisation (medical/surgical/neurosurgical), days spent in the ICU, body mass index (BMI), obesity (BMI>30), day on which MASD appeared, area (intergluteal cleft/gluteus/perineum/genitals/inguinal) and type (IAD, ID and EACD, taking into consideration the three types of MASD that can occur in the nappy area). We also monitored the number of stools and the their characteristics according to the Perineal Assessment Tool scale12 (PAT) (formed/soft/liquid), the use of a urinary catheter and, where there was spontaneous urination, the number of times this occurred, the presence of incontinence (urinary/faecal/mixed), the recording of zinc oxide application (yes/no/not necessary), the way of applying the nappy (open/closed) and the spread of fungus. In order to assess the patients’ capacity to perceive and report discomfort due to incontinence, the Glasgow Coma Scale was used. It was deemed that patient with a score of 14–15 was conscious and able to communicate. For the same purpose, with patients that were sedated, it was deemed that those given a 0 score on the Richmond Agitation Sedation Scale (RASS) were able to communicate their discomfort. Therefore, patients with a Glasgow score of ≤13 or a RASS score of anything but 0 were deemed unable to effectively communicate.
Lastly, the pressure ulcer risk score according to the Braden scale13 and the moisture exposure subscale (Fig. 1) were recorded.
Furthermore, the IAD incidence rate was calculated according to the incontinent population group.6 Urinary Incontinence (UI) is defined as involuntary urine leakages and Bowel Incontinence as the inability to control the escape of gas and faeces through the anus, characterised by the involuntary leakage thereof.14 Continent patients showing lesions due to exposure to faeces were not included in the IAD group. The incidence rate for ID and EACD was calculated out of the total sample.
Sample calculationBearing in mind that, in 2013, the number of hospitalisations with a stay of over two days in the ICU where the study was conducted was 400 patients, and assuming that the IAD incidence rate was at 30%, with 5% precision and 95% confidence interval, and establishing the amendment to adjust the number of people required, the size of the sample was set at 179 patients.
Statistical analysisIn order to analyse the data, descriptive statistics with averages (standard deviation) and ratios were used. The Kolmogorov–Smirnov test was used to find out the distribution of the quantitative variables. The variables with non-normal distribution were shown as median and percentiles, 25 and 75.
Bivariable analysis was conducted for IAD and ID since these were the most relevant types of MASD in the nappy area. Fisher's Exact Test was used for qualitative variables and Student's t-test or the nonparametric Mann–Whitney U-test were used for quantitative variables.
The Logistic Regression model was used with a lesion variable (yes vs no) as the dependent variable and the independent variables were those that showed a value of p≤0.10 in the bivariable analysis. The Stepwise regression model was used.
The Statistic Significance Level was deemed to be p≤0.05. The analysis was conducted using SPSS® software, version 19 for Windows®.
The study was approved by the Ethics Committee and the Clinical Research Committee at the institution and it was not deemed necessary to obtain informed consent since this was an observational study that did not involve any differential interventions on the patients included.
Results145 patients were included and the variables recorded are listed in Table 1.
Variables studied in the patients included in the study.
| No. | 145 |
|---|---|
| Age (year) | |
| Median [P25–P75] | 69 [56.5–76] |
| Sex | |
| Men n (%) | 96 (66.2) |
| Women n (%) | 49 (33.8) |
| Days spent in the ICU | |
| Median [P25–P75] | 5 [3–11.3] |
| BMI (kg/m2) | |
| Average (SD) | 27.5 (5.7) |
| Obesity (BMI>30): n (%) | 41 (29.9) |
| Diagnosis on admission: n (%) | |
| Medical | 90 (62.1) |
| Surgical | 43 (29.7) |
| Neurosurgical | 12 (8.3) |
| Urinary catheter user: n (%) | 132 (91) |
| Incontinent: n (%) | 42 (29) |
| Urinary | 9 (21.4) |
| Bowel | 33 (78.6) |
| Mixed | 1 (2.3) |
| Nappy users: n (%) | |
| No nappy | 48 (33.1) |
| Open nappy | 88 (60.7) |
| Closed nappy | 9 (6.2) |
| Braden scale (total) | |
| Average (SD) | 12.8 (2.5) |
| Patients unable to communicate: n (%) | 102 (70.3) |
SD: standard deviation; BMI: body mass index; P25–P75: percentile 25–percentile 75.
The incidence rate for MASD at the unit was 29% (CI 95%: 22.2–35.8), amounting to 42 cases.
When looking into the types of MASD in the nappy area it was found that 15.9% (CI 95%: 10.8–22.7) (23 cases) were due to ID, 26.2% (CI 95%: 15.3–41.1) (11 cases) due to IAD, 3.4% (CI 95%: 1.5–7.8) (5 cases) due to EACD. Furthermore, three patients were found with lesions caused by exposure to liquid faeces, which were included in the total sample but could not be classified. 71.4% of MASD presented as erythema and 28.6% as skin erosion.
The nurses documented 23.8% of the lesions due to moisture that were detected in the study.
Below we will describe the two types of MASD that appeared most frequently appear in the nappy area.
Intertriginous dermatitisOf the 23 cases with ID, in 18 of these lesions appeared in the intergluteal cleft, in 16 cases in the gluteus and 11 in the inguinal region. In some cases, the lesions expanded into various regions simultaneously.
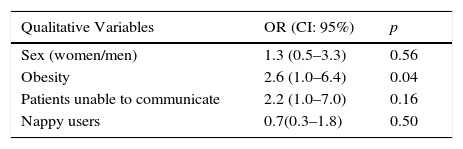
Table 2 shows the bivariable analysis regarding ID, connecting the development thereof to the same variables that were selected in the multi-variable analysis, such as the risk factors for developing ID: obesity (OR=2.8; IC 95%: 1.0–8.2) a lower score on the Braden scale (OR=0.8; IC 95%: 0.7–1.0).
Factors associated with the development of intertriginous dermatitis.
| Qualitative Variables | OR (CI: 95%) | p |
|---|---|---|
| Sex (women/men) | 1.3 (0.5–3.3) | 0.56 |
| Obesity | 2.6 (1.0–6.4) | 0.04 |
| Patients unable to communicate | 2.2 (1.0–7.0) | 0.16 |
| Nappy users | 0.7(0.3–1.8) | 0.50 |
| Quantitative Variables | ||
|---|---|---|
| Age | 0.38 | |
| Days spent in the ICU | 0.09 | |
| Braden Scale | 0.02 | |
OR: odds ratio.
Scoring was done on the moisture subscale on the Braden scale, with an average of 3.4 points (SD=0.5) in obese patients, and an average of 3.6 points (SD=0.4) in non-obese patients, p=0.08.
In 26.8% of obese patients, a barrier product was applied as a preventative measure for an average of one day [P25=1−P75=2].
The lesions that are caused by sweat or transpiration generally appeared on the 4th day [P25=2−P75=5] after hospitalisation.
Incontinence-associated dermatitisOut of the 42 incontinent patients, 11 cases of IAD were found, 9 of which were associated with BI, one with UI and one due to mixed incontinence. The lesions caused by IAD were found in the area around the anus (8 cases), the intergluteal cleft (4 cases) and the perineal area (3 cases); these were sometimes found in various areas at the same time.
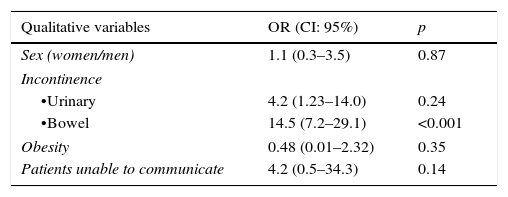
Table 3 shows the result of the bivariable analysis to detect the variables association with the development of IAD. Bowel incontinence, the number of stools made, liquid faeces and the number of days in the ICU were shown to be significantly linked to the development thereof. However, multivariable analysis selected bowel incontinence (OR=5.4; IC 95%: 1.1–26) and the number of stools made (OR=1.1; IC: 1.0–1.2) as independent factors in its development.
Factors associated with the development of incontinence-associated dermatitis.
| Qualitative variables | OR (CI: 95%) | p |
|---|---|---|
| Sex (women/men) | 1.1 (0.3–3.5) | 0.87 |
| Incontinence | ||
| •Urinary | 4.2 (1.23–14.0) | 0.24 |
| •Bowel | 14.5 (7.2–29.1) | <0.001 |
| Obesity | 0.48 (0.01–2.32) | 0.35 |
| Patients unable to communicate | 4.2 (0.5–34.3) | 0.14 |
| Quantitative variables | ||
|---|---|---|
| Days spent in the ICU | 0.01 | |
| Age | 0.28 | |
| Number of stools | <0.001 | |
| Liquid faeces | <0.001 | |
| Soft faeces | 0.32 | |
| Braden scale (total) | 0.41 | |
OR: odds ratio.
Incontinent patients were assessed according to the moisture exposure subscale with an average of 3.4 points SD=0.4) and continent patients with an average of 3.6 (SD=0.5), p=0.01.
42.9% of incontinent patients received the barrier product for an average of one day [P25=1−P75=3].
Lesions caused by IAD generally appeared on the 5th day [P25=2.75−P75=12.75] after hospitalisation.
Three patients suffering from the diarrhoea process showed lesions due to faeces exposure, but they were not deemed to be IAD since they were not incontinent.
DiscussionAccording to the results obtained, the incidence rate for MASD in the nappy area was high, reaching 29%. Until now, only lesions due to moisture in critically ill patients had been studies, which is very common and well documented. However, three types of MASD have been found in this area which is highly susceptible to the effects of moisture.
In this study, the most frequent lesion identified in the nappy area was ID, even though the incidence rate was lower than IAD, since this was calculated out of the incontinent population group. We were not able to compare this incidence rate with other studies since the literature only reports prevalence data, which is between 2 and 12%15 in patients admitted to hospitals or convalescence units and there is no data on critically ill patients.
Obesity was a predisposing factor for the development of ID. In fact, ID is a common problem in obese people16; however, it is also common in other patient groups such as women17 or children18 since this is dermatitis caused by skin-to-skin contact.
Under normal conditions, sweat tends to evaporate rapidly. However, as is the case with obese people, the transpiration that occurs in the skin folds cannot easily evaporate and the skin is exposed to this source of moisture for a longer time period.4 The over-hydrated corneal layer does not slide across the surface of the skin and causes friction leading to the lesion.
The authors of this study believe that the circumstances in which critically ill patients end up could promote this moist environment in certain areas. These types of patients are normally sedated, bed-ridden and therefore immobile and subjected to friction; these conditions could contribute towards making airflow to these areas difficult, leading to the lesion. In this respect, the Braden scale seems to have shown this, since the patients with a lower total score are at a higher risk of ID.
Furthermore, the Braden scale has a subscale which specifically assesses exposure to moisture in general terms and has been recommended by Rumbo et al.19 for assessing the risk of developing MASD. This subscale assesses exposure to faeces, urine, sweat, etc. a feature that other bedsore risk assessment scales, such as the Norton scale or the EMINA scale, which only looks at moisture in terms of incontinences, a factor that has traditionally been associated with the appearance of bedsores.20
This subscale, however, did not predict the development of ID in this study. This may be due to both in obese patients and those that were not obese, the exposure to moisture was deemed between occasionally and rarely moist, showing that sweat seems to have gone unnoticed.
The part of the nappy area where this type of lesion was most frequently seen was the intergluteal cleft, which appeared as a linear lesions that, due to its proximity to the sacrum, could be taken for a bedsore.3 In a previous study carried out at the same unit regarding differentiation between bedsores and MASD using photographs,11 it was shown that 40% would diagnose a lesion in the intergluteal cleft as a bedsore.
Although superinfections due to fungi are often common when moisture is present, none were found in this study, since the cases were closed once the lesion was detected and there was no time given for it to present.
Regarding IAD, scientific literature shows large variations in the incidence rates, moving between 3.4 and 25%.8 This variation is associated with the lack of assessment tools which means that the studies cannot be correctly compared, with the type of institution or centre, convalescence homes are predominant, with the population group studied and the difficulty in differentiating between lesions associated with dependency and more specifically bedsores and MASD.21,22
Regarding the critically ill population group, only two studies were found, both on adult patients with bowel incontinence. Driver,23 studied the incidence rate of AID comparing two cleaning procedures, the first of which was in two stages with a cleaner and without rinsing, using a barrier product, and the other with wipes that cleaned and protected at the same time. Patients treated with the first procedure showed an incidence rate of 50%, almost double that obtained in this study, despite the bibliography showing that the use of specific products instead of water an soap promotes and respects the skin's natural microclimate.24 With the second procedure, the patients benefited from this, and the incidence rate fell to 19%.2 Bliss et al.25 found an incidence rate of 36% in 45 patients with bowel incontinence from the ICUs without a definitive system for skin care and IAD prevention. Lesions appeared on the 5th day, at the same time as they appeared in the patients in this study.
The factors that predisposed them to AID were multifactorial, the most recent review identifies 27 causes,26 with the most common being urinary incontinence, bowel incontinence, the frequency of excretions and liquid faeces.25,27
In this study, we selected bowel incontinence and the number of stools as risk factors, the latter multiplying the risk of developing IAD by five. Most patients were using urinary catheter, which meant that there was only one case of urinary incontinence. In the Bliss study,25 however, frequent liquid faeces and low levels of conscience were deemed as predisposing factors.
To identify individuals at risk of AID, there is the PAT scale, which takes into consideration important factors such as the duration of the exposure, the type of irritant, the condition of the perineal skin and other factors that contribute towards the patient having liquid stools such as low albumin, pathogen infections in the intestine or enteral feeding, inter alia.12 However, as happens with most scales for predicting the pressure ulcer risk,28 the PAT scale related the exposure of the skin to moisture with the presence of urine or faeces, therefore, just one type of MASD.
Some of the items assessed on the PAT scale have been associated with the development of IAD in this study, such as the number of excretions and their consistency. However, we have not found any studies about the use of PAT in intensive care, making this a field for further study.
As previously stated in the methodology, the Braden scale was used to assess exposure to moisture in the nappy area. Even though the scores provided by the nurses between continent and incontinent patient was statistically significant, we do not believe is it is relevant in practice, since scores of occasionally and rarely humid were given to all patients, regardless of whether they presented AID or not. This score may be related to an incorrect evaluation of the moisture subscale, since the use of devices such as urinary catheters, or high rates of constipation in critically ill patients,29,30 may lead us to underestimate such exposure.
Regarding the lesions caused by faeces in three continent patients as a result of contact with faeces and repeated cleaning: it was not possible to include them in any category. These were patients in the diarrhoea process with a large number of excretions. By definition, the term AID does not allow for this type of lesions to be classified therein. This situation is also not differentiated from the other types of the MASD currently described by the GNEAUPP. It would either therefore be necessary to define a new type of MASD or to take this situation into consideration to be able to include this patient profile into one of the existing groups.
Lastly, EACD was the third type of MASD found in this study. Torra i Bou et al.5 define this as dermatitis caused by exudate that does not come from the wounds, but rather from the areas of intact skin affected by oedema. Critically ill patients often suffer from severe oedemas in the most serious stage of their illness and although the incidence rate of this lesion has been low in this study, this find is interest since it had not been previously described in these patients.
MASD prevention, specifically for AID, is based on a structured skin-care programme focused on three principles: cleanliness, hydration and skin protection.6,31 Since the ICU under study did not have a preventative programme, only the third step was recorded and analysed: the application of a barrier product, since was the only measure recorded on the medical records. In the analysis it was see that this was only used on less than half of incontinent patients, a quarter of obese patients and it was used for less time than required. This fact may show that there is a lack of awareness regarding MASD, possibly because nurses do not know about them, as shown by the small percentage of patients with MASD (23.8) shown in the chart. In the past, we have always spoken about the prevention and treatment of bedsores, and the existing evidence about assessment, diagnosis, prevention and treatment of MASD has only appeared in the last 10 years.3,4,22
Limitations of the studyThe sample was calculated according to AID, since there were no references for the incidence rate of MASD in general, nor any other specific type of moisture damage.
Given the large number of lesions that appeared and the concerns of the nursing staff at the unit, the incidence rate was calculated before reaching the required sample size. When the results were obtained, it was decided to halt data collection in order to carry out corrective measures, so the study was slightly wound down and the results must be taken with a certain degree of prudence. Due to the average amount of days spent at the unit where the study was conducted, it was decided to limit the data collection to 14 days so as to streamline statistical analysis. This may have led to the loss of cases but the study show an average of 5 days hospitalisation and the lesions appearing between the 4th and 5th day, so the effects thereof are mitigated.
ConclusionsThere is a high incidence rate of MASD in the nappy area. Nurses only recorded a quarter of the cases.
The risk factors detected for AID are bowel incontinency and the number of stools. Obesity and a low score on the Braden scale predispose patients to ID.
Incontinence is not the only source of moisture to which the patient is exposed. Sweat also plays an important role in developing these lesions.
The moisture subscale on the Braden scale is the only scale that allows us to assess exposure to different sources of moisture, making it interesting to explore whether correct scoring would make it possible to predict patients with a high risk of developing MASD just with this scale.
Less than half the population group exposed to moisture received the barrier product for a time of less than half of that required, showing that it was clearly insufficient and therefore improvable.
This study brings to light the need to raise awareness among nurses and train them regarding MASD, to improve both the recording and the means of prevention.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments have been carried out on human beings or on animals for this research.
Data protectionThe authors declare that none of the patients’ data appears in this article.
Right to privacy and informed consentThe authors declare that none of the patients’ data appears in this article.
Conflict of interestsThe authors declare that they have no conflicts of interests.
Please cite this article as: Valls-Matarín J, del Cotillo-Fuente M, Ribal-Prior R, Pujol-Vila M, Sandalinas-Mulero I. Incidencia de lesiones cutáneas asociadas a la humedad en una unidad de cuidados intensivos. Enferm Intensiva. 2017;28:13–20.