Botulism is a rare disease in Europe, caused by the bacterium Clostridium botulinum, notifiable, non-transmissible person-to-person and potentially fatal (between 5% and 10%) if not treated quickly. The favorable opinion of the Clinical Research Ethics Committee was obtained. We present the nursing care plan of a 49-year-old man with a diagnosis of bacterial intoxication caused by Clostridium botulinum, secondary to ingestion of beans in poor condition, who was admitted to the ICU for a total of 35 days.
Diagnosis and planningHolistic nursing evaluation during the first 24h, with prioritisation of the systems that were deteriorating fastest: neurological and respiratory. Nine diagnoses were prioritised according to the NANDA taxonomy: risk for allergy response, ineffective breathing pattern, impaired oral mucous membrane, impaired physical mobility, risk for disuse syndrome, risk for dysfunctional gastrointestinal motility, impaired urinary elimination, risk for acute confusion and risk for caregiver role strain.
DiscussionThe nursing care plan, standardised and organised with the NANDA taxonomy and prioritised with the outcome-present state-test (OPT) model, guaranteed the best care based on evidence, as the NOC scores improvement demonstrated. It was impossible to compare the nursing intervention with other case reports.
El botulismo es una enfermedad poco frecuente en Europa, causada por la bacteria Clostridium botulinum, de declaración obligatoria, no transmisible de persona a persona y potencialmente mortal (entre un 5 y 10%) si no se trata rápidamente. Se obtuvo el dictamen favorable del Comité de Ética de Investigación Clínica. Se presenta el proceso de cuidados enfermero de un varón de 49 años con diagnóstico de intoxicación bacteriana por Clostridium botulinum, secundario a la ingesta de alubias en mal estado, que estuvo ingresado en la UCI un total de 35 días.
Diagnósticos y planificaciónValoración enfermera de forma holística durante las primeras 24h, con priorización de los sistemas que presentaron un deterioro más rápido: el neurológico y el respiratorio. Se priorizaron 9 diagnósticos según la taxonomía NANDA: riesgo de respuesta alérgica, patrón respiratorio ineficaz, deterioro de la mucosa oral, deterioro de la movilidad física, riesgo de síndrome de desuso, riesgo de motilidad gastrointestinal disfuncional, deterioro de la eliminación urinaria, riesgo de confusión aguda y riesgo de cansancio del rol del cuidador.
DiscusiónEl proceso de cuidados enfermero, estandarizado y organizado con la taxonomía NANDA y priorizado con el método sistemático AREA, garantizó los mejores cuidados basados en la evidencia y prueba de ello fue la mejoría de las puntuaciones de los indicadores de resultado NOC. Resultó imposible comparar la actuación enfermera con la de otros casos documentados.
Botulism is a disease caused by Clostridium botulinum, an anaerobic gram-positive bacillus formed by spores that produce a powerful neurotoxin.1 It is notifiable, not transmissible person-to-person and potentially fatal (between 5% and 10%) if it is not treated promptly.2
The spores produced by the C. botulinum bacteria, which have been found in green beans, spinach, mushrooms, ham, sausages, tinned tuna and fish (fermented, salted and smoked2), germinate in anaerobic environments, and when they grow in certain environmental conditions create neurotoxins.1,2 They are heat resistant (they can survive at more than 100°C for 5h or more, but if they are exposed to 120°C for 5min they are destroyed1). They do not develop in acid media (although a pH<4.6 will not degrade an already existing neurotoxin1) and a low temperature and salt content can prevent their growth.2
C. botulinum is found all over the world and its growth depends on environmental factors.3 Van Ermengem isolated the bacteria in 1897 from a badly-cured ham. Leuchs, in 1910, demonstrated that 2 strains of C. botulinum would produce toxins with different antigenicities and in 1919 Burke named them as type A and B, thus establishing their current alphabetical designations. Subsequently, 5 further types of toxins were discovered (C, D, E, F and G), some with dual toxicities.4 Types A, B, E and occasionally F can cause human botulism; A is used cosmetically.1–3
There are 6 types of human botulism1,2: food-borne (through ingestion of foods contaminated during their preparation, processing or packaging1–3), infant (through ingestion of the spores that colonise the intestinal tract and release the toxin1–3), wound (generally due to injection of black tar heroin1–3), adult intestinal colonisation (the toxin is produced in vivo in the infected intestinal tract1), inhalation (very rare, as an act of bioterrorism1,2) and iatrogenic (through incorrect treatment1).
Between 2007 and 2015, cases of food botulism were notified in some countries in Europe and North America. According to the World Health Organisation, approximately 35% were serious, with a mortality rate of 15%, and the disease lasted from 5 to 180 days. The age mode was 50 (minimum age of 4 and maximum of 88) and 48% were males.5 In Spain, according to the Carlos III Health Institute, the autonomous communities with the highest incidence of food botulism were Castile and Leon, Andalusia and Madrid, with 20, 15 and 10 cases, respectively, although they do not specify the severity.6
In food botulism the neurotoxins, created by the digestive enzymes after C. botulinum has been ingested, pass into the blood stream and interrupt the release of acetylcholine, causing a nerve block1,2 and descending flaccid paralysis develops in the motor and autonomic nerves.2 Symptoms appear between 12 and 36h after ingestion and are principally neurological2 and gastrointestinal1,2: fatigue,2 neck muscle,2 respiratory muscle1,2 and lower limb weakness,2 vertigo,2 blurred vision,1,2 diplopia,1 drooping eyelids,1 photophobia,3 symmetric cranial neuropathy2 (speech and swallowing difficulty and dry mouth), vomiting, diarrhoea, constipation and abdominal inflammation.2
Diagnosis is based on clinical history, physical examination1 and confirmed by samples (faeces or wound, blood or food2,3). There will be no alterations in consciousness1 or haemodynamic alterations, fever or sensory deficit.2 Differential diagnosis will consider Guillain-Barré syndrome,1,2 myasthenia gravis1,2 and cerebral vascular accident.2
Treatment consists of administering the botulin antitoxin as promptly as possible,1–3 monitoring for signs of hypersensitivity because it is equine-derived,1 and overcoming the neuromuscular complications caused by the poisoning (respiratory failure is the primary cause of death1). Antibiotherapy is only indicated for wound botulism.1–3
Description of the caseA 49-year-old male, 1.72m tall, weighing 75.9kg (body mass index 25.66kg/m2). Allergic to pyrazolones, active smoker (1 pack/day). No medical history. Self-employed (Barthel Index 100).
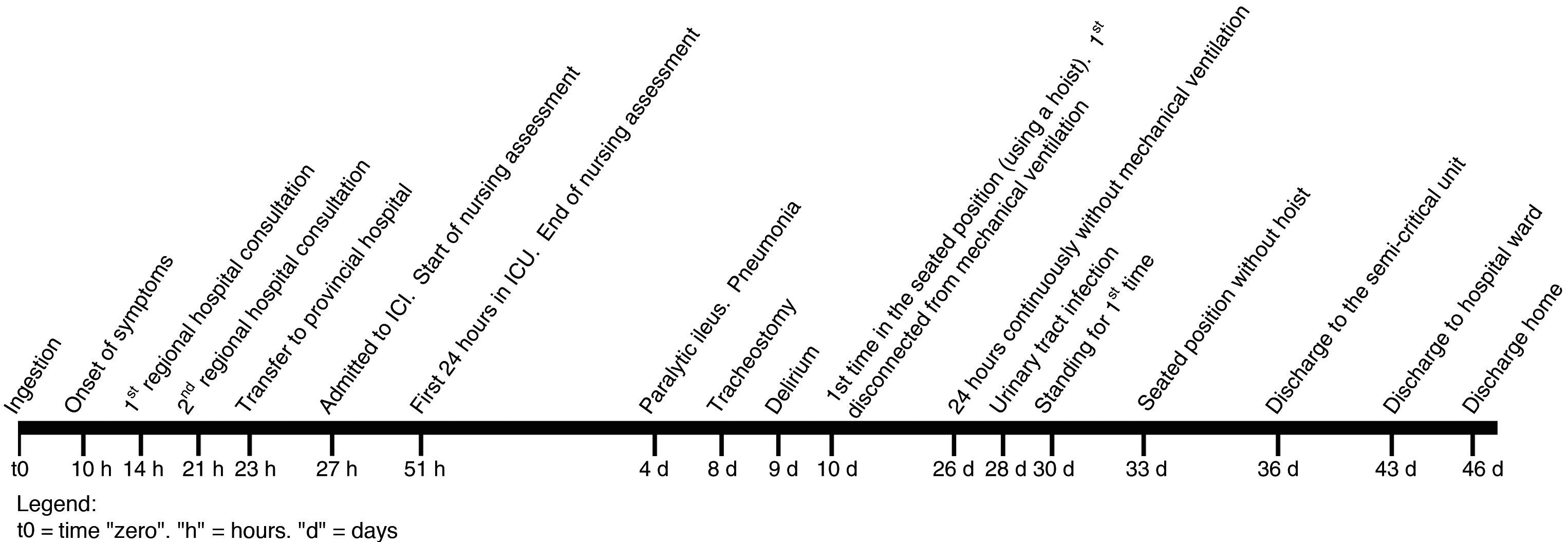
The patient attended his regional hospital with dizziness, blurred vision, unstable gait and frontal headache, of 4-h onset. He mentioned having eaten an uncertain amount of beans in poor condition 14h previously. On examination his blood pressure (BP) was 160/100mmHg. After 3h he was discharged diagnosed with bacterial food poisoning (International Classification of Disease [ICD] 005.9). Fig. 1 shows the time frame of the progression of the disease from ingestion to discharge.
He attended the emergency apartment again with drooping eyelids in addition to the abovementioned symptoms. The patient had a dry mouth, mydriatic pupils with slow photomotor reflex, bilateral drooping eyelids, and full loss of eye motion. BP 156/99mmHg. Arterial blood gases (ABG): SaO2 96.6%, pO2 78.7mmHg. There were no alterations in consciousness, cutaneo-plantar and osteotendinous reflexes or on the electrocardiogram.
The patient was transferred to the emergency department of the provincial hospital with suspected C. botulinum food poisoning (ICD 005.1). The patient now had mild dysarthria in addition to his previous symptoms. BP 139/98mmHg. ABG: SaO2 96.4%, pCO2 34mmHg, pO2 82mmHg. Peak expiratory flow (PEF, indicator of lung function, measured by spirometer) 450l/min (expected value for his height, age and sex: 601±48l/min7). Oxygen therapy was started through nasal cannula and 3h later he was admitted to the intensive care unit (ICU). He was given a first dose of the trivalent botulin antitoxin (A, B, E) by continuous infusion through a single line, and for 4h.
Nursing assessmentDuring the first 24h in ICU a holistic assessment was made of the patient that highlighted the neurological and respiratory system alterations. Bilateral facial paralysis was identified, neck muscle alteration, proximal upper limb weakness and increased dysarthria. The impact of the neuromuscular block on the respiratory system required orotracheal intubation and mechanical ventilation, due to dyspnoea and increased respiratory effort. The PaO2/FiO2 ratio, indicator of pulmonary oxygen diffusion calculated by ABG, considers there to be acute lung injury below 300mmHg8: in only 5h it had dropped from 300mmHg to 220mmHg and the PEF fell from 220l/min to 130l/min.
In addition to the neurological and respiratory alterations, and after having administered a total of 2 doses of the antidote, the patient was distressed and nauseous (with preserved peristalsis). A nasogastric tube was passed, and a urinary catheter inserted which revealed oliguria (<400ml/24h) and a central jugular venous catheter to administer vasoactive drugs, analgesia, sedation and saline.
Over the subsequent 34 days, the patient presented complications associated with intoxication and his stay in ICU. He was diagnosed with unspecified hypotension (ICD 458.9), pneumonia caused by other gram-negative bacteria (ICD 482.83), urinary tract infection, site not specified (CIE 599.0) and paralytic ileus (ICD 560.1). An early tracheostomy was performed after 7 days to enable weaning and start rehabilitation.
The patient was in ICU for a total of 35 days, 7 in a semi-critical unit and 3 on the hospital ward.
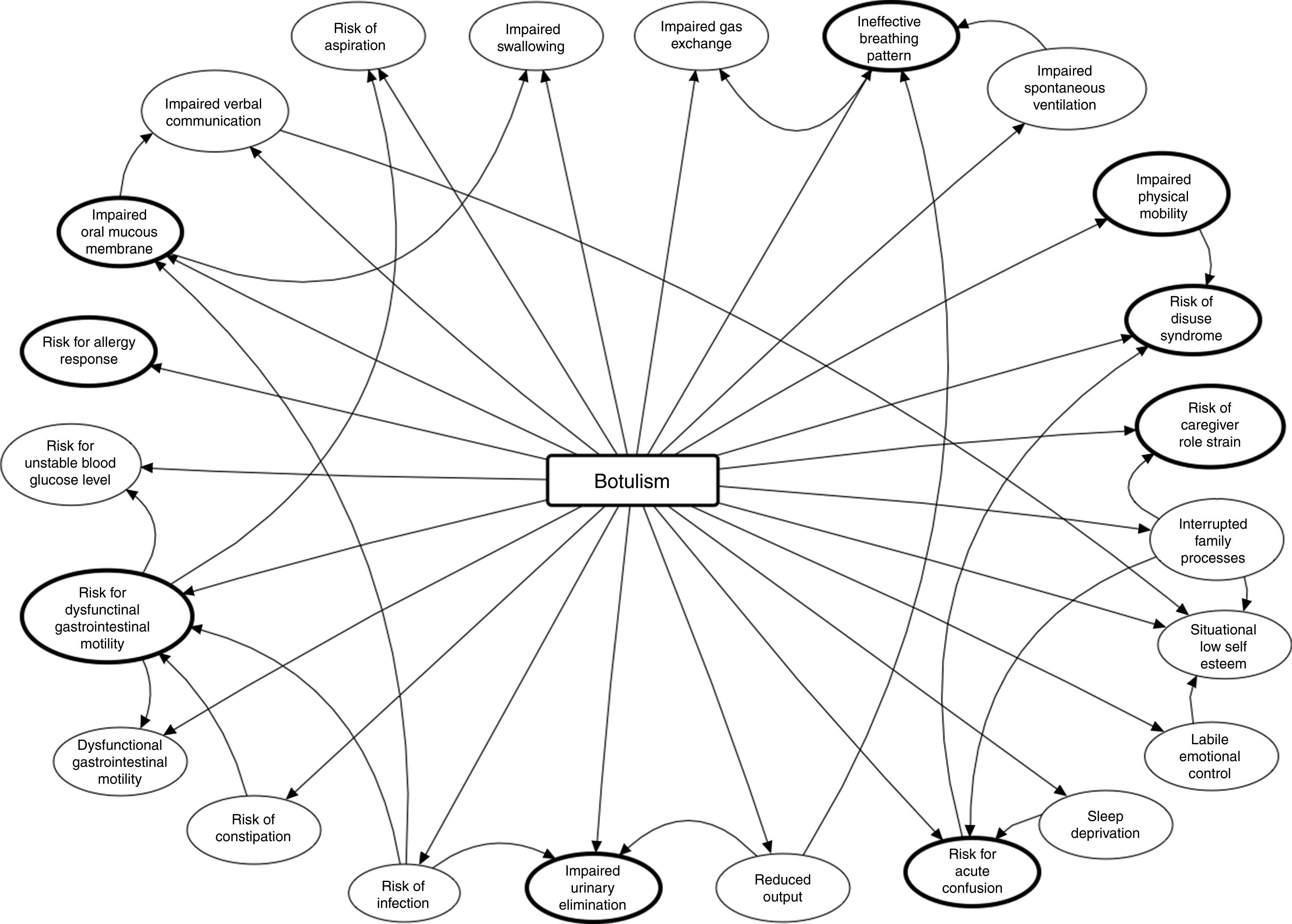
Diagnoses and care planning (NANDA-NOC-NIC)The main problem was approached by employing the OPT model of clinical reasoning,9 focussing intervention on outcomes and not on the health problems identified. The nursing objective was to anticipate the problems of progression of the patient's neuromuscular paralysis that was found on assessment. Fig. 2 shows the nursing clinical reasoning net, as created by Pesut and Herman in 1999.9 It shows the main problem, the nursing diagnoses (ND) initially identified and their interrelations. Note that 9 of them are those that eventually formed the care process (Table 1): these are the most important because if not resolved, the other 14 would persist (except for the risk of allergy, which derived directly from administration of the antidote).
Diagnosis, outcome objectives, interventions and nursing activities according to NANDA-NOC-NIC.
| Diagnosis (NANDA) | Outcome objectives (NOC) | Nursing interventions (NIC) |
|---|---|---|
| 00217 Risk for allergy response, associated with exposure to allergens (equine-derived botulism antitoxin) | 0707 Immune hypersensitivity response | 6680 Vital signs monitoring |
| 070703 allergic reactions (5, scale n) | Monitor blood pressure, pulse, temperature and respiratory status, as appropriate | |
| 6410 allergy management | ||
| Identify known allergies (medication, food, insect, environmental) and usual reaction | ||
| Monitor patient for reactions to new medications, formulas, foods, latex and/or test dyes | ||
| Monitor the patient after exposure to agents known to cause allergic responses for signs of generalised flush, angioedema, urticaria, paroxysmal coughing, severe anxiety, dyspnoea, wheezing, orthopnoea, vomiting, cyanosis or shock | ||
| 3350 Respiratory monitoring | ||
| Monitor for noisy respirations such as crowing or snoring | ||
| Monitor rate, rhythm, depth and effort of respirations | ||
| 00032 ineffective breathing pattern, related to neuromuscular impairment and respiratory muscle fatigue, manifested by reduced vital capacity | 0415 Respiratory status | 3350 Respiratory monitoring |
| 041503 Depth of inspiration (2, scale b) | Monitor oxygen saturation levels continuously in sedated patients (SaO2) per agency policy and as indicated | |
| 041509 Pulmonary function tests (1, scale b) | Monitor results of pulmonary function tests, particularly vital capacity, maximal inspiratory force, forced expiratory volume in 1s (FEV1), and FEV monitor 1/FVC, as available | |
| Monitor for dyspnoea and events that improve and worsen it | ||
| 3320 Oxygen therapy | ||
| Administer supplemental oxygen as ordered | ||
| Monitor the effectiveness of oxygen therapy (pulse oximetry, ABGs), as appropriate | ||
| 0402 Respiratory status: gas exchange | 5820 Anxiety reduction | |
| 040203 Dyspnoea at rest (3, scale n) | Monitor the patient's anxiety in relation for the need for oxygen therapy | |
| 040205 Restlessness (3, scale n) | ||
| 040208 Partial pressure of oxygen in arterial blood (PaO2) (4, scale b) | ||
| 0008 Fatigue: Disruptive Effects | 3120 Airway Insertion and Stabilisation | |
| 0803 Reduced Energy (2, scale n) | Assist with insertion of an endotracheal tube by gathering necessary intubation and emergency equipment, positioning patient, administering medications as ordered, and monitoring the patient for complications during insertion | |
| Instruct patient and family about the intubation procedure | ||
| Monitor mechanical ventilator readings, noting increases in inspiratory pressures and decreases in tidal volume, as appropriate | ||
| 3180 Artificial Airway Management | ||
| Maintain inflation of the endotracheal/tracheostomy cuff at 15–25mmHg during mechanical ventilation and during and after feeding | ||
| Institute endotracheal suctioning, as appropriate | ||
| Monitor secretions colour, amount, and consistency | ||
| Institute measures to prevent spontaneous decannulation: secure artificial airway with tape or ties, administer sedation and muscle paralysing agent, use arm restraints, as appropriate | ||
| 00045 Impaired oral mucous membrane, related to reduced salivation and manifested by dry mouth | 1100 Oral hygiene | 1730 Oral Health Restoration |
| 110010 Moisture of oral mucosa and tongue (2, scale a) | Monitor condition of the patient's mouth (tongue, mucous membranes), including character of abnormalities | |
| Administer mouthwash (antibacterial solution) | ||
| Apply lubricant to moisten lips and oral mucosa, as needed | ||
| 1720 Provide oral care for unconscious patient using appropriate precautions | ||
| Encourage and assist patient to rinse mouth | ||
| 00085 Impaired physical mobility related to neuromuscular involvement, as manifested by limited ability to perform gross and fine motor skills | 0208 Mobility | 0226 Exercise therapy: muscle control |
| 020802 body positioning performance (4, scale a) | Assist patient to sitting/standing position for exercise protocol, as appropriate | |
| 020803 Muscle movement (4, scale a) | Encourage the patient to self-exercise | |
| 020809 Coordination (4, scale a) | Monitor the emotional, cardiovascular and functional response of the patient to the exercise protocol | |
| Provide positive reinforcement for patient's efforts in exercise and physical activity | ||
| Assist patient to participate in stretching exercises when lying, sitting, or standing | ||
| Assist patient to move to sitting position, stabilise trunk with arms placed at the side on bed/chair, and rock trunk over supporting arm | ||
| 0212 Coordinated movement | 0224 Exercise therapy: joint mobility | |
| 021203 Speed of movement (2, scale a) | Assist patient to optimal body position for passive/active joint movement | |
| 021204 Smooth movement (2, scale a) | Perform passive or assisted range of movement exercises | |
| 021205 Control of movement (2, scale a) | Encourage to sit in bed, on side of bed, or in chair | |
| 021207 Balanced movement (4, scale a) | Provide positive reinforcement for performing joint exercises | |
| 0210 Transfer performance | 1806 Self-care assistance: transfer | |
| 021001 Transfer from bed to chair (1, scale a) | Determine current ability of patient to transfer self (e.g., mobility level, limitations of movement, endurance, ability to stand and bear weight, medical or orthopaedic instability) | |
| Provide encouragement to patient as he/she learns to transfer independently | ||
| Determine amount and type of assistance needed | ||
| Lift and move patient with hoist | ||
| Evaluate patient at end of transfer for proper body alignment, nonocclusion of tubes, wrinkled linens, unnecessarily exposed skin, adequate patient level of comfort, raised side rails, and call bell within reach | ||
| 0005 Activity tolerance | 0180 Energy management | |
| 000502 Pulse rate with activity (5, scale a) | Monitor cardiorespiratory response to activity (e.g., tachycardia, other dysrhythmias, dyspnoea, diaphoresis, pallor, haemodynamic pressures, respiratory rate) | |
| 000508 Respiratory rate with activity (4, scale a) | ||
| 00040 Risk for disuse syndrome, related to paralysis | 0204 Immobility consequences: physiological | 5612 Teaching: prescribe exercise |
| 020412 Muscle tone (1, scale a) | Inform the patient of the purpose for, and the benefits of, the prescribed exercise | |
| Teach the patient how to do the prescribed exercise | ||
| Reinforce information provided by other health care team members, as appropriate | ||
| 0201 Exercise promotion: strength training | ||
| Collaborate with family and other health professionals (e.g., activity therapist, exercise physiologist, occupational therapist, recreational therapist, physiotherapist) planning, teaching, and monitoring a muscle training programme | ||
| 0914 Neurological status: Spinal Sensory/Motor Function | 2620 Neurological monitoring | |
| 091401 Head and shoulder movement (2, scale a) | Monitor pupillary size, shape, symmetry, and reactivity | |
| 091405 Upper body strength (2, scale a) | Monitor level of consciousness | |
| 091406 Flaccidity (3, scale n) | Monitor level of orientation | |
| 0912 Neurological status: consciousness | Monitor trend of Glasgow coma scale | |
| 091201 Opens eyes to external stimuli (1, scale a) | Monitor cough and gag reflex | |
| 91213 Delirium (2, scale n) | Monitor facial symmetry | |
| Monitor EOMs and gaze characteristics | ||
| Monitor for visual disturbance: diplopia, nystagmus, visual-field cuts, blurred vision, visual acuity | ||
| 00197 Risk for dysfunctional gastrointestinal motility, related to ingestion of contaminated foods | 0501 Bowel elimination | 2300 Medication administration |
| 050101 Elimination pattern (1, scale a) | Monitor patient for the therapeutic effect of the medication | |
| 050129 Bowel sounds (2, scale a) | Document the administration of medication and the patient's response capacity, per agency protocol | |
| 2380 Medication management | ||
| Monitor patient for the therapeutic effect of the medication | ||
| 1015 Gastrointestinal function | 1570 Vomiting management | |
| 101501 Food tolerance (1, scale a) | Measure or estimate emesis volume | |
| 101510 Amount of residual gastric aspirates (1, scale a) | Ensure effective antiemetic drugs are given to prevent vomiting, when possible | |
| 101530 Gastric reflux (1, scale n) | Monitor fluid and electrolyte balance | |
| 1874 Tube care: gastrointestinal | ||
| Check that the tube is correctly in place monitoring for signs and symptoms of tracheal positioning, checking the colour and and/or pH of aspirate, inspecting the oral cavity and/or verify placement by X-ray, if appropriate | ||
| Initiate and monitor delivery of enteral tube feedings, as appropriate per agency protocol | ||
| Monitor amount, colour, and consistency of nasogastric output | ||
| Monitor for sensations of fullness, nausea, and vomiting | ||
| Connect tube to suction, as appropriate | ||
| 1056 Enteral tube feeding | ||
| Check residual every 4–6h for the first 24h, then every 8h during continuous feedings | ||
| Check residual before each intermittent feeding | ||
| Hold tube feedings if residual is greater than 150 cc or more than 110–120% of the of the hourly rate in adults | ||
| 1200 Total parenteral nutrition administration | ||
| Encourage a gradual transition from parenteral to enteral feeding, if indicated | ||
| 2301 Medication response | 1080 Gastrointestinal intubation | |
| 230101 Expected therapeutic effects (3, scale a) | Administer medication to increase peristalsis, if indicated | |
| 00016 Impaired urinary elimination, related to sensory-motor impairment, as manifested by oliguria | 0503 Urinary elimination | 4120 Fluid management |
| 050303 Urine amount (2, scale a) | Maintain accurate intake and output record | |
| 0601 Fluid balance | Insert urinary catheter, if appropriate | |
| 060107 24-hour intake and output balance (3, scale a) | Administer prescribed diuretic drugs, if appropriate | |
| Administer prescribed I.V. therapy | ||
| 4130 Fluid monitoring | ||
| Determine possible risk factors for fluid imbalance (diuretic therapy, infection) | ||
| Examine skin turgor by pinching the skin gently holding it for a second and releasing (skin will fall back quickly if patient is well hydrated) | ||
| Monitor serum and urine electrolyte values, as appropriate | ||
| Monitor BP, heart rate, and respiratory status | ||
| Keep an accurate record of intake and output (enteral intake, IV intake, antibiotics, fluids given with medications, NG tubes, vomit) | ||
| Monitor colour, quantity, and specific gravity of urine | ||
| 00173 Risk for acute confusion aguda, related to: | 0901 Cognitive orientation | 4820 Reality orientation |
| – pharmacological agents | 90101 Identifies self (5, scale a) | Address patient by name when initiating interaction |
| – disorders of sleep-wake cycle | 90102 Identifies significant other (5, scale a) | Speak to the patient in a distinct manner with an appropriate pace, volume, and tone |
| – impaired mobility | 90103 Identifies current space (5, scale a) 90105 Identifies correct month (5, scale a) | Inform patient of person, place, and time, as needed |
| Engage the patient in concrete “here and now” activities (ADL) that focus on something outside the self that is concrete and reality oriented | ||
| 5270 Emotional support | ||
| Discuss with the patient the emotional experience | ||
| Make supportive or empathetic statements | ||
| 6430 Chemical restraint | ||
| Monitor the patient's response to the medication | ||
| Monitor level of consciousness | ||
| 5820 Anxiety reduction | ||
| Provide factual information concerning diagnosis, treatment, and prognosis | ||
| Identify when level of anxiety changes | ||
| Help patient identify situations that precipitate anxiety | ||
| Administer medications to reduce anxiety, as appropriate | ||
| 7560 Visitation facilitation | ||
| Determine patient's preferences for visitation and release of information | ||
| Determine the need to promote visits by family and friends | ||
| Encourage the family member to use touch, as well as verbal communication, as appropriate | ||
| 00062 Risk for caregiver role strain, related to unpredictable illness course (applied to the family) | 1803 Knowledge: disease process | 7040 Caregiver support |
| 180302 Characteristics of specific disease (2, scale u) | Determine caregiver's level of knowledge | |
| 180303 Cause and contributing factors (1, scale u) | Determine caregiver's acceptance of role | |
| 180305 Physiological effects of disease (2, scale u) | Acknowledge difficulties of caregiving role | |
| 180307 Usual course of disease process (2, scale u) | Acknowledge dependency of patient on caregiver (as appropriate) | |
| 180309 Potential complications of disease (3, scale u) | Make positive statements about caregiver's effort | |
| Provide support for decisions made by caregiver | ||
| Provide information about patient's condition in accordance with patient preferences | ||
| Identify sources of respite care | ||
| Support caregiver in setting limits and taking care of self |
Taken from NNNConsult.10
Table 1 combines the ND (NANDA10), outcomes (NOC10) and nursing interventions (NIC10), with their respective outcome and activity indicators. Table 2 shows the assessment scales of the different outcome indicators.10
Likert scales to assess outcome indicators.
| Scale a |
| Severely compromised |
| Substantially compromised |
| Moderately compromised |
| Mildly compromised |
| Not compromised |
| Scale b: |
| Severe deviation from normal range |
| Substantial deviation from normal range |
| Moderate deviation from normal range |
| Mild deviation from normal range |
| No deviation from normal range |
| Scale n: |
| Severe |
| Substantial |
| Moderate |
| Mild |
| None |
| Scale u |
| No knowledge |
| Limited knowledge |
| Moderate knowledge |
| Substantial knowledge |
| Extensive knowledge |
Taken from NNNConsult.10
Bearing in mind the natural course of the disease, outcome indicators (NOC) with deficient scores were inevitable. The thorough neurological monitoring, the deterioration in breathing over the first 24h, the early tracheostomy and the persistent nursing action on rehabilitation were noteworthy.
DiscussionThe case we present confirms the need for good coordination between practitioners, in terms of communication, intervention and recording, since the patient made good progress thanks to the rapid action of the multidisciplinary team.
The nursing care process comprised a holistic assessment that flagged up the involvement of the neurological and respiratory systems, and action based on strict monitoring of the course of the disease and the constant moral and physical support of the patient.
The use of standardised language (NANDA-NOC-NIC) and systematic use of the OPT model of clinical reasoning enabled the nursing intervention to be organised and prioritised, ensuring the best care based on current scientific evidence. We found no literature relating to the nursing care of patients with botulism in ICU; therefore it is impossible to compare our action with that of other authors.
Ethical responsibilitiesProtection of people and animalsThe authors declare that the research was carried out according to the ethical standards set by the responsible human experimentation committee, the World Medical Association and the Helsinki Declaration.
Data confidentialityThe authors declare that they have followed the protocols of their centre of work regarding the publication of patient data.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects referred to in the article. This document is held by the corresponding author.
Conflict of interestThe authors have no conflict of interest to declare.
We would like to thank all the professionals who documented, provided and contributed towards the compilation of information.
Please cite this article as: Zariquiey-Esteva G, Galeote-Cózar D, Santa-Candela P, Castanera-Duro A. Botulismo en la UCI: proceso de cuidados. Enferm Intensiva. 2018;29:86–93.