To identify commonly used intravenous drugs that may produce endothelial damage.
MethodsAn experimental research study was performed using a sample of 62 intravenous drugs commonly used in emergency care, pH and osmolarity were measured. Subsequently, based on these values, the theoretical capacity to cause irritation or endovascular damage was determined and classified as high, moderate, and low.
ResultsSamples from 19 drugs for fluid therapy, 21 antibiotics and 22 drugs for intravenous use were studied. Glucose solutions, sodium bicarbonate 1M and mannitol 10% showed a high capacity to cause venous irritation. Vancomycin, ciprofloxacin, amiodarone, haloperidol, and labetalol solution presented a high capacity for irritation based on their acidic pH. The antibiotics, dexketoprofen, diazepam, digoxin, etomidate, phenytoin, levetiracetam and metamizole also showed high osmotic values in their reconstituted or undiluted presentations. Moreover, osmolarity of diazepam, digoxin and phenytoin remained high despite being diluted in 100 ml of saline.
ConclusionsKnowing the pH and osmolarity of intravenous drugs allows their capacity to cause endothelial damage to be assessed. The use of comprehensive tables based on the chemical properties of the drugs can be a useful tool to help prevent chemically-induced phlebitis.
Identificar los medicamentos intravenosos de uso común en el ámbito hospitalario con capacidad de producir daño endotelial.
MétodoEstudio experimental in vitro. La muestra estuvo formada por 62 medicamentos de uso común en los servicios de urgencias y hospitalización. Las variables estudiadas fueron: osmolaridad y pH. Posteriormente, en base a esos valores, se determinó la capacidad teórica para provocar daño endotelial, clasificándola en alta, moderada y baja.
ResultadosSe analizaron 19 medicamentos para fluidoterapia, 21 antibióticos y 22 medicamentos intravenosos. Las soluciones de glucosa, el bicarbonato 1M y el manitol 10% presentaron una capacidad elevada para provocar irritación venosa. La vancomicina, ciprofloxacino, amiodarona, haloperidol y labetalol mostraron una capacidad irritativa elevada derivada de su pH marcadamente ácido. Los antibióticos, el dexketoprofeno, diazepam, digoxina, etomidato, fenitoína, levetiracepam y metamizol presentaron valores extremos de osmolaridad en su presentación reconstituida o sin diluir y mantuvieron sus valores de tonicidad elevados después de diluirlos en 100 ml de suero salino el diazepam, la digoxina y la fenitoína.
ConclusionesConocer el pH y osmolaridad de los medicamentos intravenosos permite evaluar su capacidad para provocar daño endotelial. La creación de tablas comprensivas en base a las propiedades químicas de los medicamentos puede constituir una herramienta útil que contribuya a prevenir la flebitis químicamente inducida.
Chemically induced phlebitis is an inflammatory response of the vascular endothelium to the intravenous administration of irritating drug solutions. It is a common complication in hospitalised patients receiving intravenous therapy via peripheral catheters.
What it contributesKnowing the physicochemical properties of intravenous drugs, such as pH and osmolarity, helps in the assessment of their capacity to cause venous irritation or endothelial damage.
Implications of the studyThe creation of comprehensive tables based on the physicochemical properties of drugs may be a useful tool which, together with the assessment of other factors, can help prevent chemically induced phlebitis by taking appropriate measures for vascular access care.
IntroductionPhlebitis is a common complication in hospitalised patients receiving intravenous therapy via peripheral catheters. An estimated 30% of patients with a peripheral venous catheter may develop phlebitis.1 Depending on its aetiology, phlebitis can be classified as infectious, mechanical, or chemical.
Chemically induced phlebitis is an inflammatory response of the vascular endothelium to the intravenous administration of irritating pharmacological solutions. Irrespective of other factors, the onset of chemical phlebitis has been related to the administration of intravenous antibiotic therapy or other irritant solutions,2–4 primarily determined by their chemical characteristics, such as osmolarity (which defines the number of particles per litre of solution) and pH (i.e., the degree of acidity or alkalinity of the solution).
Blood plasma has a pH between 7.35 and 7.45 and an osmolarity of approximately 290 mOsm/l. The incidence of phlebitis increases if the pH and osmolarity of the intravenous solution differ from those of blood. Fluids with higher osmolality and solutions with acidic or alkaline pH cause irritation and endothelial damage, leading to phlebitis and even thrombus formation in severe cases.5
Nurses are the professionals responsible for the preparation and administration of parenteral drugs, but not all the conditions that can lead to phlebitis are always well known in the non-specialist clinical setting.6 In fact, detailed information on the chemical characteristics of intravenous drugs that can be used to identify their potential to cause endothelial damage is scarce and often outdated or decontextualised.
Nurses are also the primary managers of venous capital for patients admitted to hospital. They must choose the most appropriate type of catheter depending on the characteristics of the intravenous drug to be administered, the duration of treatment and the clinical condition of the patient. In addition to professional judgement, scientific evidence and clinical decision support tools provide quality assurance in vascular access care.7
The aim of this work was to identify intravenous drugs commonly used in the hospital setting with the potential to cause endothelial damage due to their chemical characteristics.
MethodDesign, sample, and scope of the studyAn experimental in vitro study was conducted in an independent laboratory at the University of the Basque Country.
The researchers selected a sample of 62 medicines commonly used in hospitals. The drugs were selected by consensus between the researchers and three nurses from a tertiary hospital, ensuring a representative and equitable sample of serotherapy drugs, antibiotics, and other drugs administered most commonly in emergency departments or hospitalisation units. The drugs studied were marketed in Spain as of November 2020.
Study variablesThe pH and osmolarity of different intravenous drugs under different dilution forms were determined.
Data collectionThe pH and osmolarity values were determined using an osmometer (Fiske, model 210) and a pH meter with automatic temperature compensation system (Mettler-Toledo, model SevenCompact). To determine pH, a single measurement of an aliquot of each sample was taken at room temperature (20 ± 1 °C). For osmolarity, two measurements were performed, recording the highest reading. The measuring devices were calibrated before each measurement according to the manufacturer’s recommendations.
For antibiotics, the osmolarity and pH of the drug diluted in 100 ml of compatible serum were analysed: .9% saline (pH5.7; 280 mOsmol/l). In cases where direct intravenous administration was indicated, osmolarity measurements of the reconstituted principle in 10 ml of .9% saline were also performed. Wherever available, generic pharmaceutical products were used.
For the remaining drugs, the osmolarity of the undiluted solution was measured, and for those indicated for intermittent infusion, the osmolarity and pH of the diluted drug were determined in 50–100 ml of .9% saline or 5% glucose saline (pH 4.3; 289 mOsmol/l), in case of incompatibility.
As a general rule, the concentration of the dilutions was in line with the usual concentration for clinical use. Dilutions were prepared according to the instructions provided on the product label.
Data analysisBased on the information obtained regarding pH and osmolarity, each drug’s theoretical capacity to cause venous irritation or endothelial damage of each drug was determined according to the following classical classification8:
- •
High: pH < 4.5 or >9 and/or >500 mOsm/l (extreme if >900 mOsm/l).
- •
Moderate: pH between 4.5–6.9 or 8–9 and/or 350–500 mOsm/l.
- •
Low: pH between 7.0–7.9 and/or <350 mOsm/L.
The information is presented in three differentiated comprehensive tables for solutions intended for fluid therapy, antibiotics, and other drugs.
ResultsSamples from 19 commercial solutions for fluid therapy, 21 antibiotics and 22 intravenous drugs commonly used in emergency and inpatient departments were analysed in the laboratory.
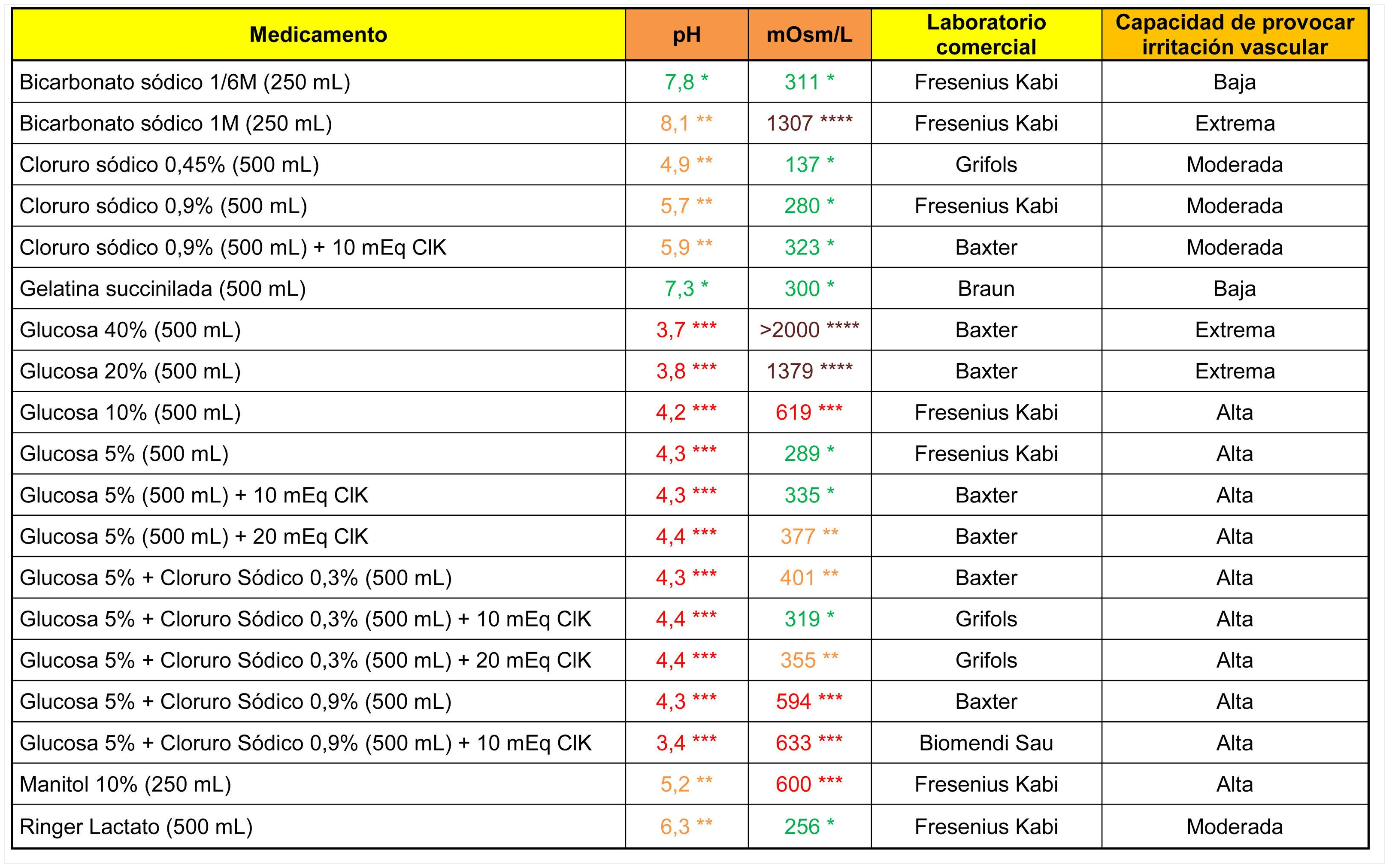
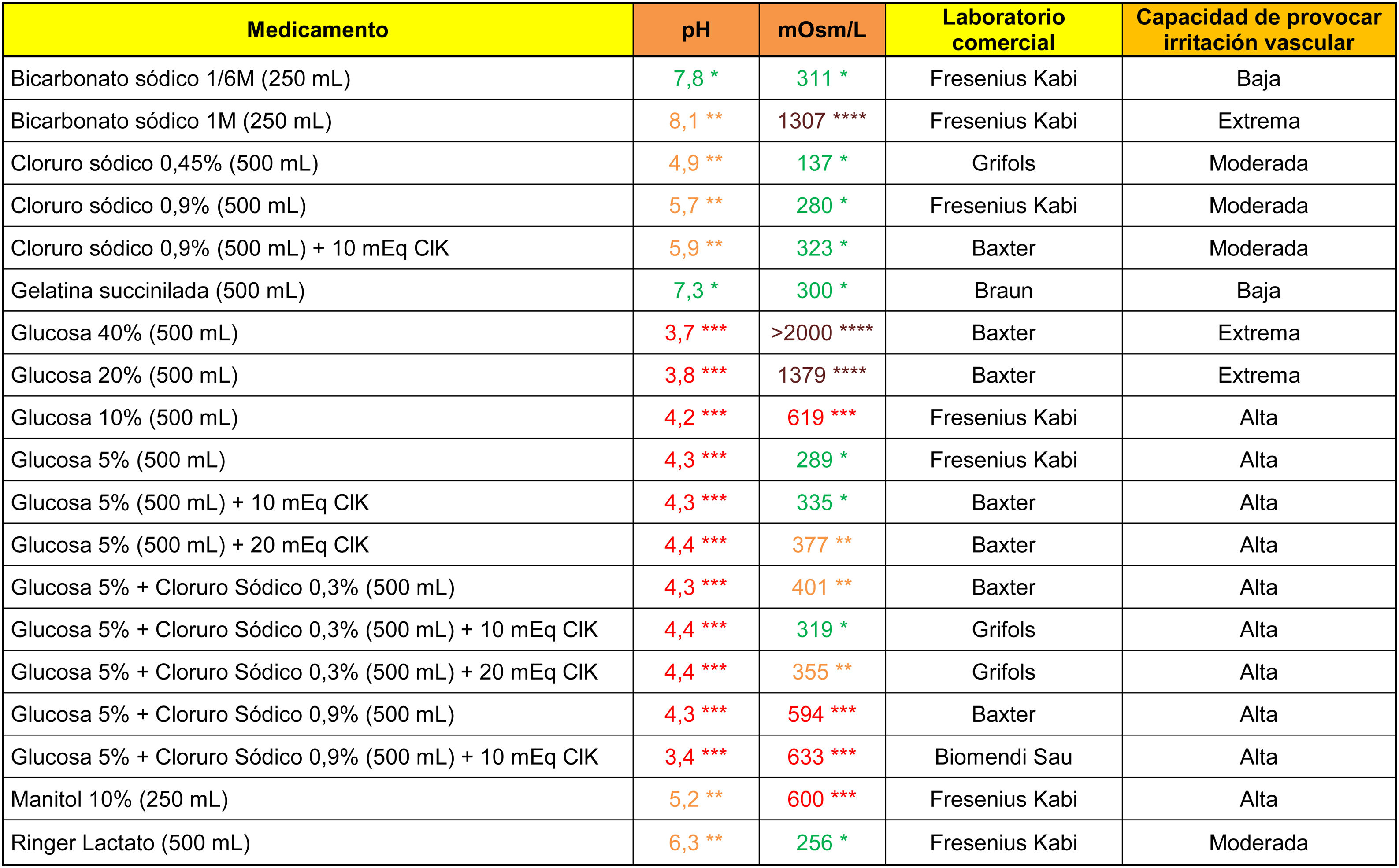
The analytical data of commonly used fluid therapy solutions are shown in Table 1. All the glucose solutions analysed showed an acidic pH (below 4.5). Glucose solutions above 5%, glucosaline, 10% mannitol and 1M bicarbonate showed a high capacity to cause venous irritation due to their hypertonic properties.
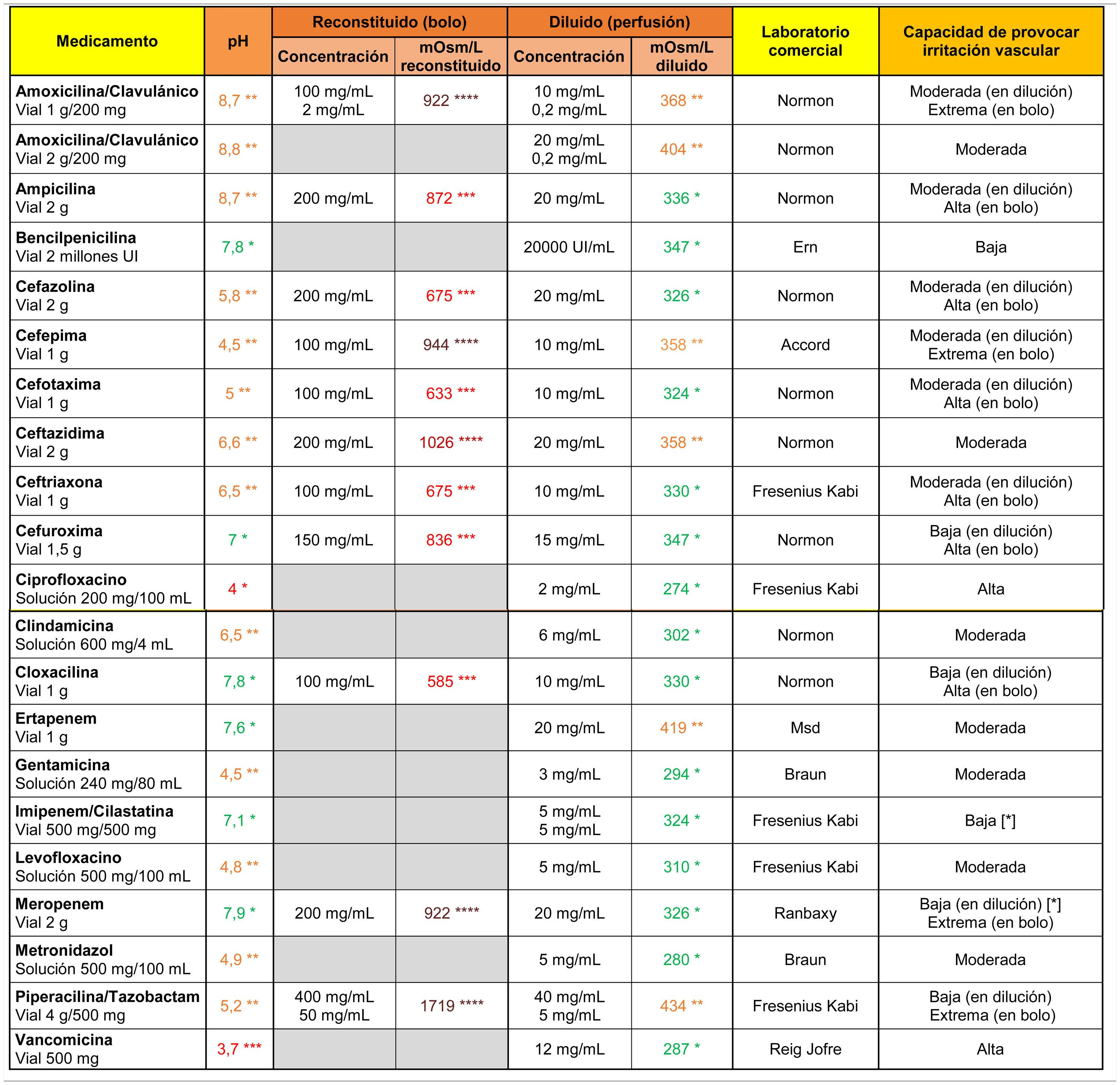
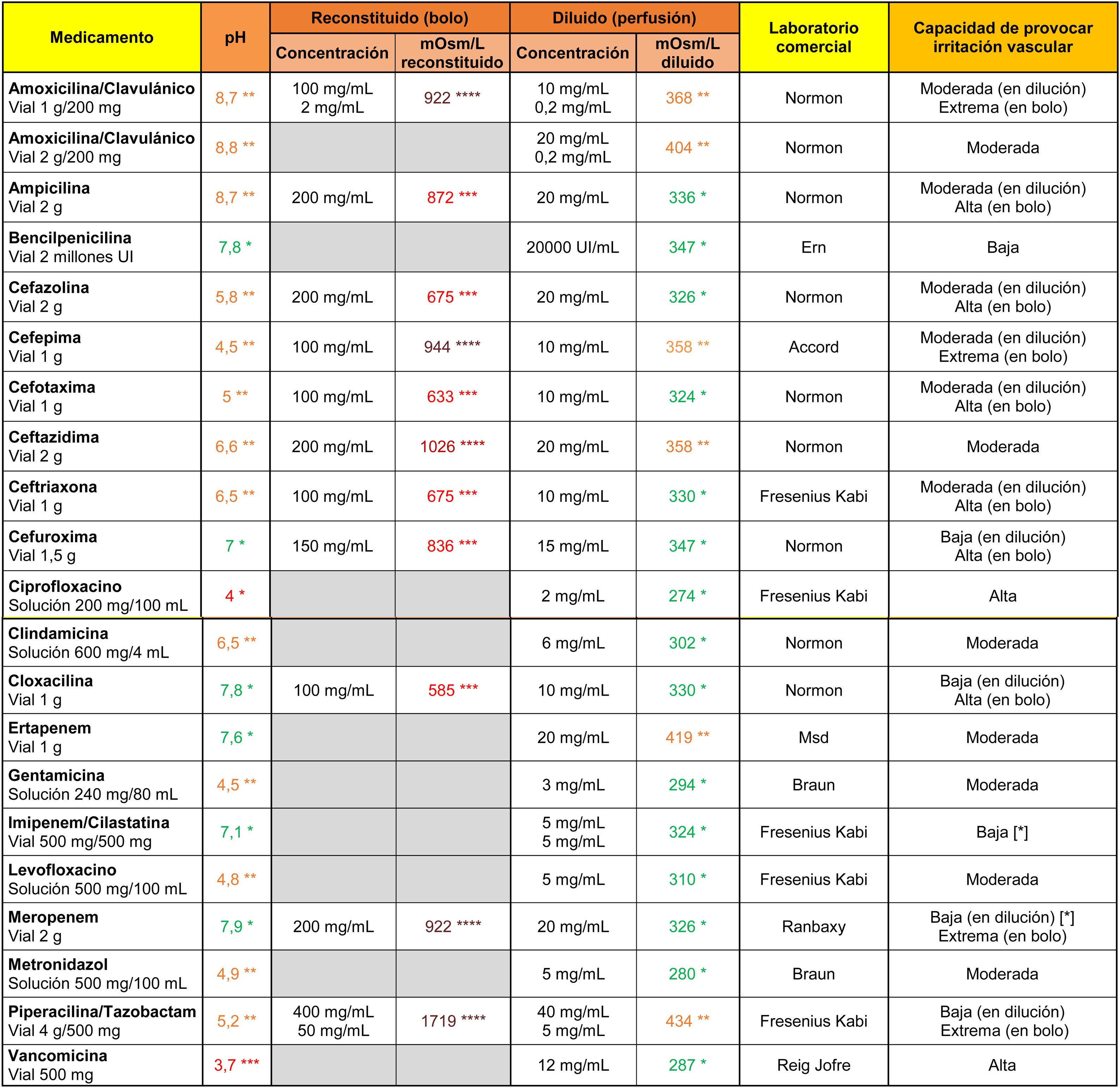
In the case of antibiotics (Table 2), vancomycin and ciprofloxacin showed a high irritant capacity related to their markedly acidic pH. All antibiotics indicated for direct intravenous administration were highly hypertonic when reconstituted in 10 ml of .9% saline. Their ability to cause vascular irritation decreased to moderate or low values when diluted in 100 ml saline.
Osmolarity (mOsm/l), pH and theoretical capacity to cause vascular irritation of different intravenous antibiotics.
[*] Drugs with the potential to cause endothelial damage due to their toxic characteristics, irrespective of pH and osmolarity values.
****Brown: >900 mOsm/l; ***Red: pH < 4.5 or >9 or 500–900 mOsm/l; **Orange: pH in ranges 4.5–6.9 or 8–9 or 350–499 mOsm/l; *Green: pH in range 7.0–7.9 or <350 mOsm/l.
Cells in grey: administration not recommended under this presentation.
Note 1. The powder vials were reconstituted with 10 ml of .9% saline. Only the osmolarity of the reconstituted product was calculated for antibiotics indicated for direct intravenous administration.
Note 2. The drugs were diluted in .9% saline. It is possible to calculate the theoretical osmolarity that would have resulted from reconstitution with distilled water by subtracting the osmolarity value of the.9% saline from the value shown in the table.
Data obtained in the laboratory.
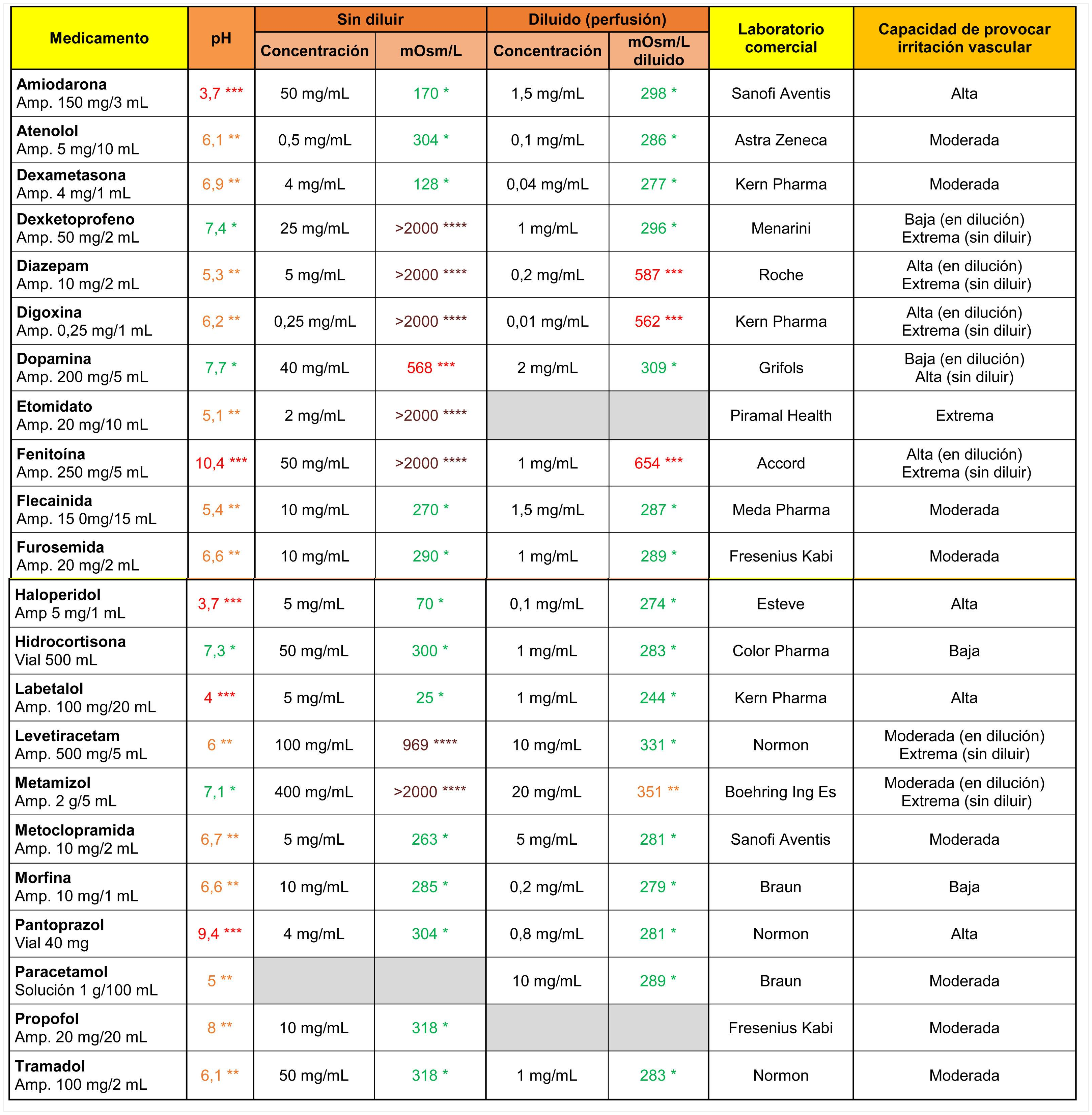
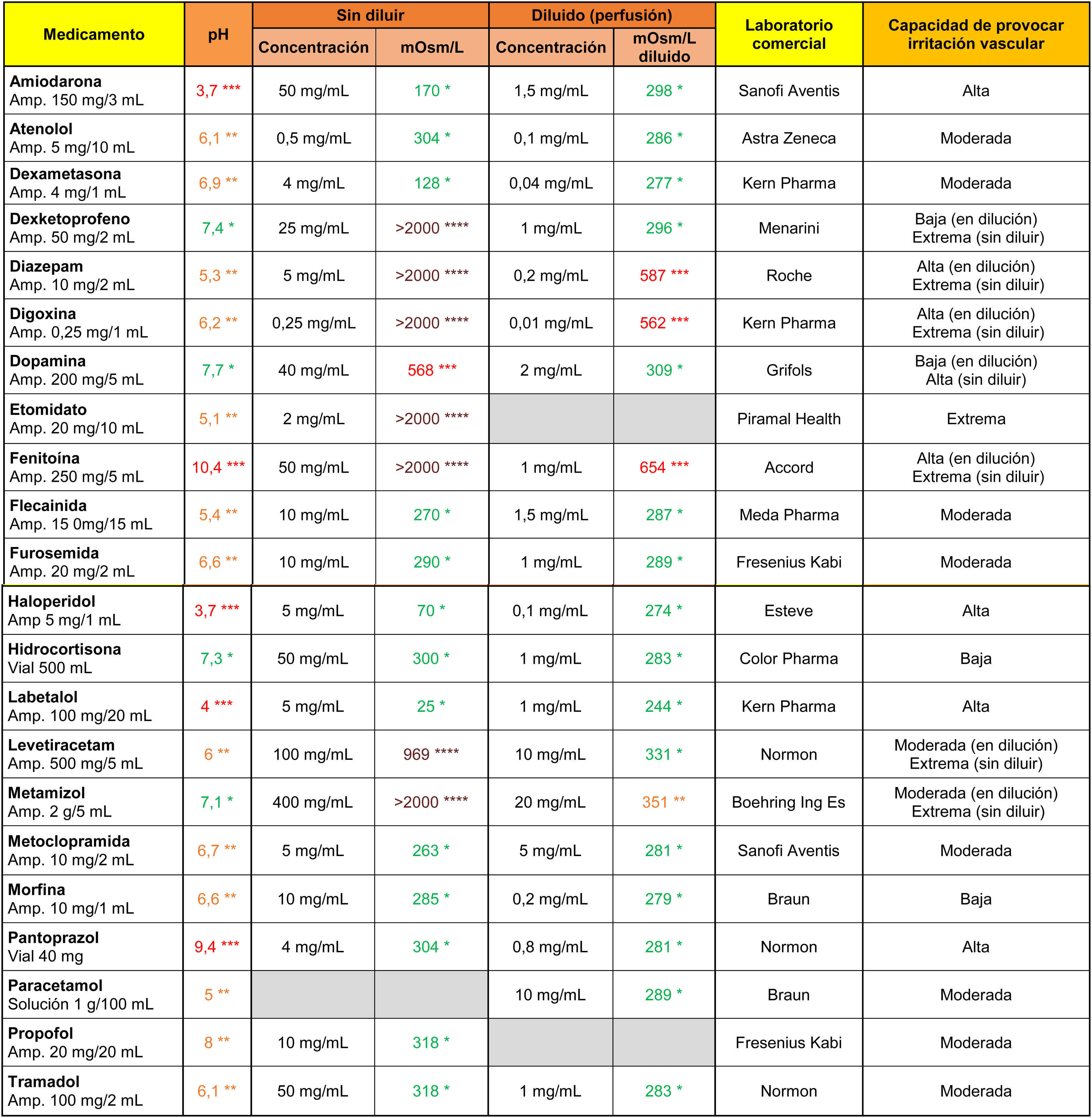
Amiodarone, haloperidol, and labetalol stood out from the other drugs (Table 3) for their markedly acidic pH, and phenytoin and pantoprazole for their alkalinity. On the other hand, dexketoprofen, diazepam, digoxin, etomidate, phenytoin, levetiracetam and metamizole showed extreme osmolarity values in their original presentation (undiluted), and diazepam, digoxin and phenytoin maintained their high tonicity values after dilution in 100 ml saline.
Osmolarity (mOsm/l), pH and theoretical capacity to cause vascular irritation of different intravenous drugs.
****Brown: > 900 mOsm/l; ***Red: pH < 4.5 or >9 or 500–900 mOsm/l; **Orange: pH in ranges 4.5–6.9 or 8–9 or 350–499 mOsm/l; *Green: pH in range 7.0–7.9 or <350 mOsm/l.
Cells in grey: administration not recommended in this presentation.
Amp: ampoule.
Note 1. The powder vials were reconstituted with 10 ml .9% saline.
Note 2. The drugs were diluted in .9% (except for amiodarone and labetalol, which were diluted with 5% glucose saline).
Data obtained in the laboratory.
This study made it possible to identify the capacity of some of the most common peripheral intravenous drugs administered in the hospital setting to cause irritation and/or endothelial damage. In addition to the clinical condition of the patient and the indications for treatment, determining the pH and osmolarity values of these drugs provides useful information for deciding the form of administration or the most appropriate venous access device, with the aim of reducing the risk of chemically induced phlebitis.
The results of this work are consistent with the findings of other similar larger studies conducted in parallel with drugs marketed in Spain.9 Different trials based on laboratory analyses with different drugs, preparations and dilutions help to create a still poorly developed risk map of chemically induced phlebitis.
Occasionally, drug data sheets provide data on osmolarity and pH. In the past, other authors have stratified the risk of chemical phlebitis of various drugs based on the information provided in the drug manufacturer’s data sheets.10 Although the information from these studies has been used to make recommendations and warnings about their administration in clinical practice,11,12 caution is required in making decisions based on these data for several reasons. Firstly, because these studies were conducted more than a decade ago and outside our geographical context, where the commercial brands and, therefore, the physicochemical characteristics of the drugs and their excipients may differ from the current formulations developed in Spain. And, secondly, because the information on pH given in the technical data sheets corresponds to the range of values for which a product has been authorised and in which its efficacy, safety and quality can be certified, but does not represent the exact value of the solution. Furthermore, the osmolarity data provided by the datasheets (when supplied) refer to the undiluted drug. This last point is relevant, as it is common (if not necessary) for drugs to be reconstituted or diluted so that they can be administered correctly. In this case, dilution of the drug in a compatible liquid can lead to significant changes in the final osmolarity. It would be advisable for the data on pH and osmolarity in different dilutions to be included by default in the drug data sheets. In any case, it would be more correct to refer to the capacity of drugs to cause vascular irritation or damage, and not directly to phlebitic risk, since the risk of phlebitis depends on factors in addition to the physical-chemical characteristics of the drug, such as the frequency or time of administration or the characteristics of the individual patient.
From the information obtained in this paper, from the laboratory measurements a series of recommendations can be made or highlighted to minimise the consequences of the irritant characteristics of the intravenous administration of drugs with osmolarity and/or pH values outside the optimal range, such as pain during administration or subsequent risk of vascular damage.
Osmolarity and pH of solutionsHyperosmolar solutions cause an influx of water from the interstitial and cellular spaces into the plasma and may produce local effects such as swelling, heat and pain.
To calculate the osmolarity of a solute in a solution, it is enough to calculate the number of moles and divide by the volume of solvent in litres. The number of moles in turn is calculated by dividing the grams of substance by the molecular weight of that substance. Since the osmolarity value is volume dependent, it is possible to reduce the molar concentration by increasing the amount of diluent (e.g., isotonic saline).
In parallel, a way of reducing the molar concentration in reconstituted drugs is to use a hypotonic diluent, such as distilled water for injection (0 mOsm/l). This is highly interesting for drugs to be administered as a bolus, since reconstitution of some lyophilised or powdered drugs with .9% saline can give hyperosmolar solutions, whereas they would be isotonic or slightly hypertonic if reconstituted with the same amount of distilled water. In our work we used 10ml of physiological saline solution to reconstitute the powdered drugs, replicating the technique most commonly used in the services consulted. The theoretical way to approximate the final osmolarity of the drug if it had been reconstituted with distilled water is to subtract the osmolarity value of the physiological saline (280 mOsm/l, in our case) from the value obtained. Thus, for example, and along the same lines as suggested by other authors,13 in our trial we would have observed a clinically relevant reduction in osmolarity after reconstitution of cloxacillin 1 g or cefotaxime 1 g (inter alia) if we had used distilled water instead of physiological serum to reconstitute them.
To achieve dilutions with lower osmotic load, it is recommended that antibiotics be administered by intermittent infusion, diluting the drug in at least 100 ml of compatible saline, provided there is no contraindication. When direct bolus administration is necessary, powdered, or lyophilised antibiotics should be reconstituted, where possible, with distilled water for injection.
Unlike osmolarity, pH does not vary in a clinically relevant way according to the dilution volumes commonly used, but it should be noted that the pH of a drug may differ according to the pharmaceutical presentation and the manufacturing laboratory or even the temperature of the solution.
In the laboratory data of our study, vancomycin and amiodarone stand out for their markedly acidic pH values (pH = 3.7), and are classified as having a “high capacity to cause vascular irritation”. This classification is consistent with documented clinical findings, which describe a common association between phlebitis and repeated peripheral venous administration of these drugs.14,15
However, some authors recommend that neither osmolarity nor pH as isolated parameters should be considered risk criteria for deciding placement of a central venous line,16,17 but that their concomitance and other factors, such as volume, duration and speed of perfusion, type of catheter or venous blood flow, among others, should be assessed.
Rate of drug administrationIn solutions with high osmolarity and/or pH, the duration of intravenous infusion is a major factor in venous endothelial damage.
The risk of vascular irritation is higher in prolonged infusions than in direct bolus or rapid intermittent infusions. Based on animal studies, vascular tolerance is directly related to osmolarity and duration of perfusion: the faster the administration of hypertonic solutions, the better the tolerance of the veins. For example, perfusion tolerance through peripheral veins has been identified for solutions of 880 mOsm/l administered for 8h, but only of 550 mOsm/l for 24 h.18 Similarly, rapid administrations (within minutes) of small volumes of solutions with pH out of the range (and even extreme values) did not produce clinically appreciable vascular damage.19,20
Although animal and human biology clearly differ, it seems reasonable to extrapolate that the exposure time of the vascular endothelium to irritant solutions should be reduced as far as the characteristics of the drug and the patient allow. If not possible, or if multiple doses are required, central venous catheterisation should be considered.
Choice of vessel and vascular access deviceThe blood flow rate at the tip of the catheter has been linked to the risk of chemical phlebitis. The higher the flow rate in the vein, the greater the dilution of the drug as it enters the bloodstream and, therefore, there will be less vascular damage caused.3,21,22 Since the blood flow rate in the superior vena cava is higher than in a peripheral vein, the need for a central venous catheter should be considered for continuous or medium to long-term administration of solutions with high/extreme irritation potential.5,23
Other factors related to the intrinsic characteristics of the drugThe physico-chemical structure or nature of a solution also influences vascular damage after intravenous administration. There are drugs that, despite having osmolarity and pH values within the desirable range, may cause damage to the vascular endothelium due to their toxicity (such as imipenem, meropenem or some cephalosporins, among others)24 or even the individual vascular tolerance of the patient. In addition to osmolarity and pH, the toxic characteristics of the drugs and the patient’s individual characteristics must be considered in clinical practice.
LimitationsThis work has some limitations that should be mentioned. Firstly, the list of drugs presented is not intended to be exhaustive, but rather an example for consideration. Only a sample of drugs frequently used in emergency departments and hospital units has been represented and only a few of the possible dilutions considered, and therefore the information contained in the tables of results should be interpreted with caution considering the way the drugs were prepared. Furthermore, the drugs were prepared on the assumption that they would be administered to adult patients, not taking the particularities of paediatric treatments into account.
It should also be noted that the laboratory measurements were only conducted on a single aliquot in the case of pH, and in any case on a single commercial presentation of each drug. The precision of the analysers used, and the homogeneity of the dilutions studied make it unlikely that there are clinically relevant differences between the measurements of two aliquots (they showed a difference of less than 3% in osmolarity tests). However, the same analyses on drugs from other manufacturers could have yielded different results, although these differences are also unlikely to be clinically relevant.9
Finally, it is also worth noting that the ranges used to stratify the ability to cause vascular irritation are based on consensus and expert opinion, as the exact values are unknown at which a given pH and/or osmolarity alone cause endothelial damage.
ConclusionIn light of the results of our study, we can conclude that there is a need to determine the physicochemical properties of intravenous drugs, such as pH and osmolarity, to identify drug presentations with the potential to cause endothelial damage. The creation of comprehensive tables based on the chemical properties of drugs may, together with the assessment of other factors, be a useful tool to help prevent chemically induced phlebitis by taking appropriate measures for vascular access care.
FundingNo funding was received for this paper.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank Victoria Armenteros Yeguas, for her review and contributions to this text.