Pain is a subjective experience which is assessed verbally. However, the quality of verbal expression is often questioned and/or altered for subjects with a psychiatric disorder, thus complicating access to care and pain relief with a deny of their pain. Our aim was to search for obvious behavioral signs of pain feeling in subjects with schizophrenia (SC) or major depression (MD).
MethodsSixteen MD, 17 SC, and16 disease-free participants were video-recorded during an experimental session comprised of an interview (baseline), pain test, and post-test period. Several non-verbal behavioral indicators were analyzed during the session and compared between the disease and control groups.
ResultsSome behavioral indicators were more frequent during the pain tests than during the other periods, independently from the group.
ConclusionHetero-evaluation scales for conventional pain rating should be completed by the observation of these discreet behavioral indicators of pain during painful situations. Their observation during medical examination of painful points will ensure the feeling of pain, and avoid a deny of the pain feeling for psychiatric patients.
Pain is a subjective experience. Its relief depends on the ability of the person who experiences the pain to communicate it and the recognition/understanding by those susceptible to relief it, including care givers and practitioners. The main problem in the care of pain in patients with mental disorders is the risk that what is verbally expressed does not reflect what is actually perceived. Clinical and experimental studies and observations suggest that pain sensitivity and expression are modified in mental disorders.1 Subjects with schizophrenia (SC) or other psychotic disorders are thought to be either hypoalgesic,2,3 or hyperalgesic.4 In contrast, depressed people complain more in daily life than control subjects, with the consequence that they are not trusted when they verbally express pain, although their sensitivity to pain seems to be equivalent to that of controls.5,6
Thus, it is difficult for clinicians to know exactly how patients with mental disorders perceive pain, because pain is a private and personal experience which depends of many factors. The main solution is to observe their behavior due to pain. Pain behavior is a manifestation of suffering due to pain which is produces by tissue damage (nociception). Shankland7 defines pain behavior by “anything a person says or does to reflect the presence of tissue damage”.
Pain is composed of sensory, affective, emotional, and cognitive components, all influencing behavior according to their respective importance, which can vary between specific groups or even individuals. Facial expressions of emotion, gestures, and body postures are largely involuntary. Thus, the information provided by the analysis of non-verbal behavior may differ from verbal information.8 Our interest thus focused in this study on searched for non-verbal cues of pain which would be useful for the recognition of pain in patients with psychiatric disorders.
Many pain studies have been conducted to understand the behavior of non-communicating populations or those with diminished cognitive abilities, resulting in the construction of several pain scales based on behavioral observation (elders, newborns, subject with autism, subjects in palliative care, etc.).9–11 They are based on the recognition of facial expressions and behaviors attributed to painful situations (e.g. avoidance reactions).12 Almost all the scales include items dealing with crying/wailing, corporal signs (the search of antalgic positions, stiffness), behavioral signs (ability to be comforted, interest of children in games, reaction to requests), or even physiological signs (blood pressure).13–15 They are based on the recognition of various indicators by an evaluator: either verbal or non-verbal vocal complaints, grimaces, face contractions, gripping, the search for a comfortable position, and rubbing/massage of the painful zone.
Thus, the sensory, affective, emotional, and cognitive components are modified in psychiatric diseases, but the resulting consequences on the pain-related behavior have not been studied. No specific pain scales or indicators have been identified for patients with psychiatric disorders such as SC and major depression (MD), although their expression of pain is often questioned and the quality and meaning of their oral expression are still subjected to caution. Identifying such specific behavioral signs would allow the objectivation of pain feeling in such patients during care.
Aim of the studyThe main objective of the study was to determine the frequency of behavioral indicators related to body movement, facial expression, and oral expression during the induction of experimental pain tests in a control group (without a known psychiatric disorder) and in groups of subjects with SC (SC group) or MD (MD group). We assessed the potential association between these behavioral indicators and the results of experimental pain tests evaluated by a Visual Analogic Scale (VAS) that relies on the verbal expression of pain. We used blood pressure (BP) and heart rate (HR) as stress-related autonomic measures and evaluated the intensity of anxiety, depression, and catastrophism.
Materials and methodsStudy populationAll participants were first included in a main study of which the objective was to explore pain sensitivity and experience in patients with psychiatric disorders (Clinical trial number NCT01575912).
Participants with psychiatric disordersAll subjects hospitalized at Esquirol Hospital, Limoges, France were invited to participate in the main study if they were diagnosed with SC (SC group) or had a major depressive episode (MD group) as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR)16 criteria, validated by their referent psychiatrist. The first 15 subjects for each group included in the main study were asked to participate in this pilot study and signed a specific consent form.
Free of psychiatric disease participantsSubjects without a known psychiatric history and free of any punctual antalgic or analgesic therapeutic treatment were recruited for the main study following a call for volunteers from the hospital staff, with no proposed compensation. The first 15 subjects included in the main study were asked to participate in this pilot study and signed a specific consent form.
All participants were between 18 and 60 years old, had health insurance, were not under antalgic or analgesic treatment, and gave written informed consent for participation in the study. The exclusion criteria were an inability to understand the tests or the French language, uncontrolled high blood pressure, poor blood coagulation, alteration in nociceptive capacity and discernment, nerve lesions or dermatosis of the upper extremities, lesion or muscular pathology of the upper limbs, non-treated alcohol use disorder, use of an illicit drug during the 24h before the tests, latex allergy, pregnancy, forced hospitalization, or participation in previous pain studies of the Research Department of the hospital.
The study was approved by a local ethics committee and received all legal authorization from the French authorities.
Experimental proceduresAll sessions were video recorded to register all behavioral indicators. The experiment was divided into several periods in the following order: a basal period that started at the beginning of the video recording, consisting of the time for the installation of the participant until the start of the clinical interview, giving the reference behavioral indicators for each participant; a three part testing period corresponding to the pain tests (pressure test P, and two parts for the ischemia induction test I1 and I2); and a post-test period that began at the end of the last pain test and ended at the moment of the removal of the first physiological measurement device.
Clinical evaluationsAnxiety and depression intensity was evaluated by the Hospital Anxiety and Depression Scale (HAD)17 and catastrophism using the Pain Scale Catastrophizing scale (PCS).18
The duration of hospitalization before the tests was recorded, as well as the pharmaceutic treatment (described according to the major psychotropic classes).
Pain tests and measurementsThe current level of subjective pain (present pain on the day of the tests) was evaluated using a Visual Analogic Scale (VAS) at the beginning of the session.
Pain tests corresponded to the induction of moderate pain using a procedure adapted from previous studies.4,5 The two experimenters were clinicians trained in the pain test procedure.
Briefly, a habituation test was performed before the stationary pressure application, which consisted of the application of three pressure values of 40, 80, and 160kPa. After each 15-second application, the participant was asked to evaluate the intensity of pain felt using the VAS. If the score was >2.5 at the last pressure tested, the subsequent test was performed by applying a pressure of 160kPa to the forearm. If the score was still <2.5, the subsequent test was performed by applying a pressure of 200kPa to the forearm. The number of subjects experiencing pain intensity evaluated by VAS>3 was recorded, as this threshold corresponds to the WHO threshold for first grade antalgic prescription. The completion time for the pressure test defined the P period.
The other test consisted of the induction of ischemia: a blood pressure cuff was inflated to a pressure greater than 10% of the peak systolic pressure, and the participant was asked to evaluate their arm pain each minute using the VAS. The test was stopped when the score reached 3 and the blood pressure cuff was deflated. The time required to obtain a pain score of 3 with the VAS was recorded, and corresponds to I1. The maximum time allowed for the test was 960s. Once the cuff had been deflated and removed, the test continued until the participant declared a pain equal to 0 by the VAS, corresponding to the I2 period.
The post-period test was defined as the time for the participants to return to a calm situation and remove the devices used for the experiment, similar to the basal period.
Behavioral indicator recordingThe session was video recorded by two video cameras (Sony HDR-CX 160E) to collect the indicators according to the focal-individual sampling method. One video camera was placed to film the participant's profile, with a broad view of their feet to their shoulders, and the other was positioned behind the experimenter, facing the participant, to film the face.
Stress indicators or pain behavioral indicators were selected from various existing pain scales13 that have been used in several studies involving the expression of pain.19–21 These indicators were classified into three components, and assessed during each period of the session: body component (leg movements, swinging, change of position involving mobilization of the torso or pelvis, self-centered gestures, massage of the body area concerned by pain tests), facial component (wrinkled pleated forehead, grin/wince grimace, frowns), and vocal component (breathing noises, groans or moaning, any verbalization of pain or of painful sensations such as “it stings,” “that's hot”, “it hurts”) (for more details see Appendix A1). All behavioral indicators were detected and quoted by the same examining examiner (ethologist).
The sum of the indicators of each component represents a behavioral stream that was analyzed for the different time periods of the experiment. The sum of each of the three components stream represented the total behavioral stream.
Neurovegetative measuresChanges in heart rate were measured using a heart rate monitor continuously recording the heartbeat with a computer interface (HR variation). This measure was designed to assess changes in the participants’ condition during the experimental session in relation to anxiety. Blood pressure was measured at the beginning and end of the session.
Statistical analysisNon parametric tests were used because of the non normal distribution of the variables and the small sample size. Data are described by median and inter-quartiles if quantitative, or percent and number (n) if qualitative. Non-parametric paired tests (Wilcoxon) were used to compare quantitative data during two different periods of the session. The non-parametric Mann–Whitney test was used to compare quantitative data between groups. The Chi squared test was used if needed to explore differences in the repartition of qualitative data between groups. A probability <0.05 was considered to be significant. Tests were performed using SAS software.
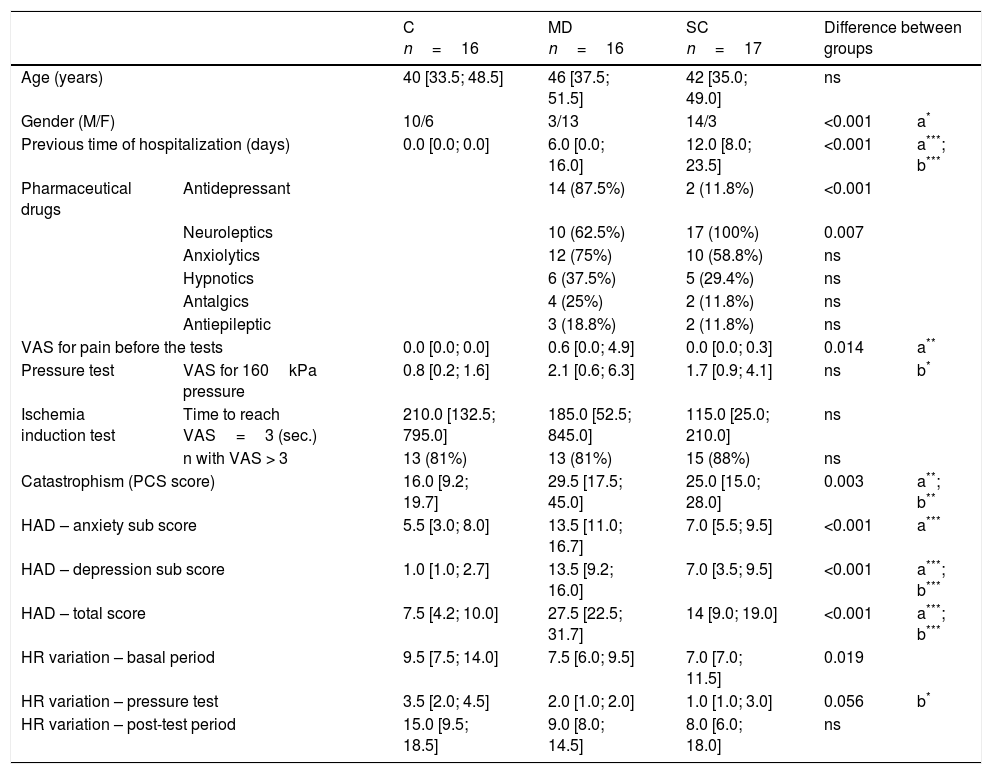
ResultsPopulationClinical, sociodemographic, and pain test data for the MD group (n=16), SC group (n=17), and controls (n=16) are presented in Table 1. Genders were unequally divided among the groups. VAS scores for pain were not high before the tests, except for some MD subjects.
Data for the control (C), major depressive episode (MD), and schizophrenia (SC) groups. Data are shown as the medians [quartiles] or frequency (percent).
| C n=16 | MD n=16 | SC n=17 | Difference between groups | |||
|---|---|---|---|---|---|---|
| Age (years) | 40 [33.5; 48.5] | 46 [37.5; 51.5] | 42 [35.0; 49.0] | ns | ||
| Gender (M/F) | 10/6 | 3/13 | 14/3 | <0.001 | a* | |
| Previous time of hospitalization (days) | 0.0 [0.0; 0.0] | 6.0 [0.0; 16.0] | 12.0 [8.0; 23.5] | <0.001 | a***; b*** | |
| Pharmaceutical drugs | Antidepressant | 14 (87.5%) | 2 (11.8%) | <0.001 | ||
| Neuroleptics | 10 (62.5%) | 17 (100%) | 0.007 | |||
| Anxiolytics | 12 (75%) | 10 (58.8%) | ns | |||
| Hypnotics | 6 (37.5%) | 5 (29.4%) | ns | |||
| Antalgics | 4 (25%) | 2 (11.8%) | ns | |||
| Antiepileptic | 3 (18.8%) | 2 (11.8%) | ns | |||
| VAS for pain before the tests | 0.0 [0.0; 0.0] | 0.6 [0.0; 4.9] | 0.0 [0.0; 0.3] | 0.014 | a** | |
| Pressure test | VAS for 160kPa pressure | 0.8 [0.2; 1.6] | 2.1 [0.6; 6.3] | 1.7 [0.9; 4.1] | ns | b* |
| Ischemia induction test | Time to reach VAS=3 (sec.) | 210.0 [132.5; 795.0] | 185.0 [52.5; 845.0] | 115.0 [25.0; 210.0] | ns | |
| n with VAS > 3 | 13 (81%) | 13 (81%) | 15 (88%) | ns | ||
| Catastrophism (PCS score) | 16.0 [9.2; 19.7] | 29.5 [17.5; 45.0] | 25.0 [15.0; 28.0] | 0.003 | a**; b** | |
| HAD – anxiety sub score | 5.5 [3.0; 8.0] | 13.5 [11.0; 16.7] | 7.0 [5.5; 9.5] | <0.001 | a*** | |
| HAD – depression sub score | 1.0 [1.0; 2.7] | 13.5 [9.2; 16.0] | 7.0 [3.5; 9.5] | <0.001 | a***; b*** | |
| HAD – total score | 7.5 [4.2; 10.0] | 27.5 [22.5; 31.7] | 14 [9.0; 19.0] | <0.001 | a***; b*** | |
| HR variation – basal period | 9.5 [7.5; 14.0] | 7.5 [6.0; 9.5] | 7.0 [7.0; 11.5] | 0.019 | ||
| HR variation – pressure test | 3.5 [2.0; 4.5] | 2.0 [1.0; 2.0] | 1.0 [1.0; 3.0] | 0.056 | b* | |
| HR variation – post-test period | 15.0 [9.5; 18.5] | 9.0 [8.0; 14.5] | 8.0 [6.0; 18.0] | ns | ||
VAS: Visual Analog Scale; PCS: Pain Catastrophizing Scale; HAD: Hospital and Depression Scale; HR: heart rate. a: comparison C/MD; b: comparison C/SC.
As already observed in previous studies, the SC and MD groups declared a higher VAS pain score for the pressure pain test than the controls, but the difference was significant only between controls and SC subjects (p=0.034). The time to reach a VAS score=3 was shorter for the SC group than for the other groups, without significance.
Catastrophism and HAD were higher in the two psychiatric groups; but the MD group had a significantly higher score for anxiety and depression than the SC group. The psychiatric groups had smaller HR variations than the control group during the experiment. The largest variations for all groups occurred during the post-test period.
Blood pressure between the arrival of the participants for testing and their departure decreased in the control (p=0.006) and SC groups (p=0.03).
HR fluctuated during pressure tests. Analysis of the variation of HR during the test showed significant differences between the groups. The variation of HR of the MD group was lower than that of the control group during the pressure (p=0.01) and the SC group was greater than that of the control group during the pressure test (p=0.005).
No association was found between pharmaceuticals drugs use and behavior indicators during the experiment.
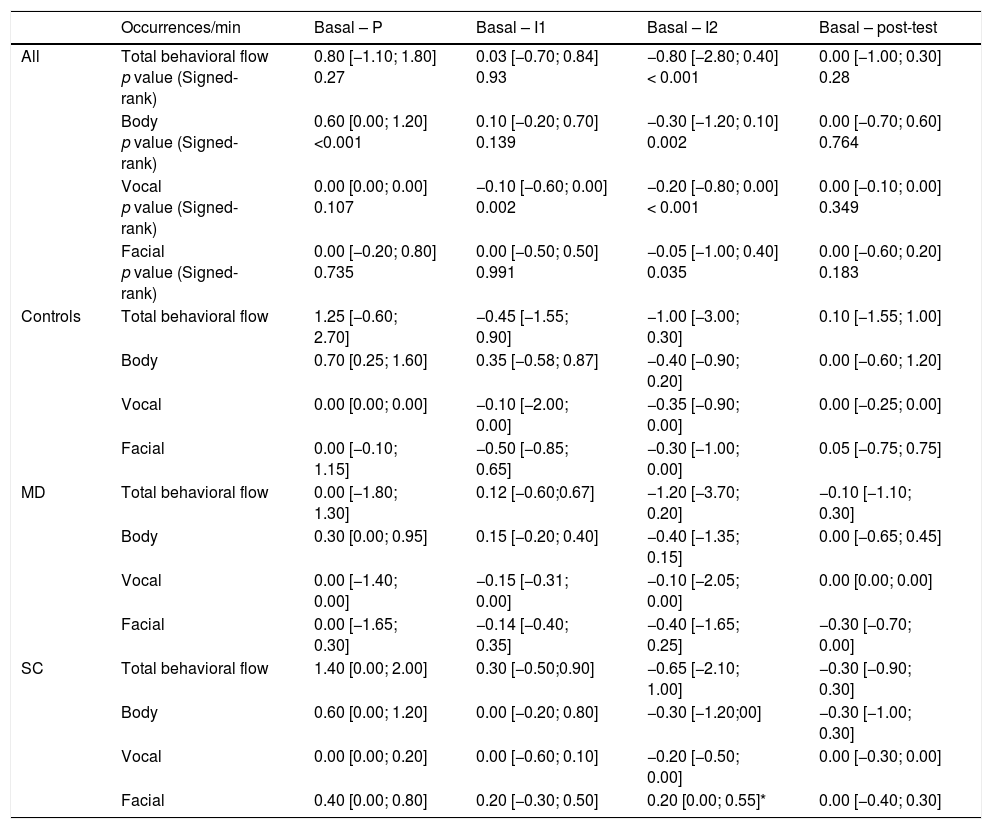
Overall behavioral streamDuring the experiment, the overall behavioral stream for all subjects varied from period to period (1.6 [0.5; 2.90]occurrences/min during the basal period, 1.3 [0.7; 3.20]occurrences/min during the test period, and 1.8 [1.1; 3.8]occurrences/min during post-test period). The specific differences in occurrence frequencies between periods are presented in Table 2.
Differences of total behavioral flow, and body, vocal, and facial flows between the basal and other periods of the session for the whole population and each group.
| Occurrences/min | Basal – P | Basal – I1 | Basal – I2 | Basal – post-test | |
|---|---|---|---|---|---|
| All | Total behavioral flow p value (Signed-rank) | 0.80 [−1.10; 1.80] 0.27 | 0.03 [−0.70; 0.84] 0.93 | −0.80 [−2.80; 0.40] < 0.001 | 0.00 [−1.00; 0.30] 0.28 |
| Body p value (Signed-rank) | 0.60 [0.00; 1.20] <0.001 | 0.10 [−0.20; 0.70] 0.139 | −0.30 [−1.20; 0.10] 0.002 | 0.00 [−0.70; 0.60] 0.764 | |
| Vocal p value (Signed-rank) | 0.00 [0.00; 0.00] 0.107 | −0.10 [−0.60; 0.00] 0.002 | −0.20 [−0.80; 0.00] < 0.001 | 0.00 [−0.10; 0.00] 0.349 | |
| Facial p value (Signed-rank) | 0.00 [−0.20; 0.80] 0.735 | 0.00 [−0.50; 0.50] 0.991 | −0.05 [−1.00; 0.40] 0.035 | 0.00 [−0.60; 0.20] 0.183 | |
| Controls | Total behavioral flow | 1.25 [−0.60; 2.70] | −0.45 [−1.55; 0.90] | −1.00 [−3.00; 0.30] | 0.10 [−1.55; 1.00] |
| Body | 0.70 [0.25; 1.60] | 0.35 [−0.58; 0.87] | −0.40 [−0.90; 0.20] | 0.00 [−0.60; 1.20] | |
| Vocal | 0.00 [0.00; 0.00] | −0.10 [−2.00; 0.00] | −0.35 [−0.90; 0.00] | 0.00 [−0.25; 0.00] | |
| Facial | 0.00 [−0.10; 1.15] | −0.50 [−0.85; 0.65] | −0.30 [−1.00; 0.00] | 0.05 [−0.75; 0.75] | |
| MD | Total behavioral flow | 0.00 [−1.80; 1.30] | 0.12 [−0.60;0.67] | −1.20 [−3.70; 0.20] | −0.10 [−1.10; 0.30] |
| Body | 0.30 [0.00; 0.95] | 0.15 [−0.20; 0.40] | −0.40 [−1.35; 0.15] | 0.00 [−0.65; 0.45] | |
| Vocal | 0.00 [−1.40; 0.00] | −0.15 [−0.31; 0.00] | −0.10 [−2.05; 0.00] | 0.00 [0.00; 0.00] | |
| Facial | 0.00 [−1.65; 0.30] | −0.14 [−0.40; 0.35] | −0.40 [−1.65; 0.25] | −0.30 [−0.70; 0.00] | |
| SC | Total behavioral flow | 1.40 [0.00; 2.00] | 0.30 [−0.50;0.90] | −0.65 [−2.10; 1.00] | −0.30 [−0.90; 0.30] |
| Body | 0.60 [0.00; 1.20] | 0.00 [−0.20; 0.80] | −0.30 [−1.20;00] | −0.30 [−1.00; 0.30] | |
| Vocal | 0.00 [0.00; 0.20] | 0.00 [−0.60; 0.10] | −0.20 [−0.50; 0.00] | 0.00 [−0.30; 0.00] | |
| Facial | 0.40 [0.00; 0.80] | 0.20 [−0.30; 0.50] | 0.20 [0.00; 0.55]* | 0.00 [−0.40; 0.30] |
Comparison: C/MD and C/SC.
The behavioral stream of all participants during the I2 period (removal of the cuff) was significantly higher than that of the basal period (p=0.0002) with a median difference of −0.80 events per minute between these two periods. The three components of behavior (body, vocal, and facial) increased significantly between the basal and I2 periods (Table 2) for the whole population. The stream of the vocal component increased significantly between the basal and I1 periods. The stream of the body component decreased very significantly between the basal and P periods (p<0.001). There was no difference between the pre-test and post-test periods for the whole population, either for entire behavioral stream nor the different behavioral components. There were also no significant differences in overall behavioral stream between the basal and I2 periods (p=0.38) or the basal and any other period between the groups. Analysis of the behavioral component streams revealed a significant difference between the SC and C groups for the facial component between the basal and I2 periods.
Behavioral indicatorsThere were significant differences between the basal period and pain test periods (P, I1 and I2) for seven behavioral indicators and a trend toward significance for the indicator “frowning” for the whole population (n=49) (Fig. 1). Behavioral indicators of the components for which we found an increase between the basal and P periods were: verbalization (p=0.008); between the basal and I1 period: massages (p=0.008), moans (p=0.031), verbalization (p<0.001); between the basal and I2 periods: grimaces (p=0.041), massages (p<0.001); moans (p=0.004); verbalization (p<0.001); and between the basal and PT periods: grimaces (p=0.016).
There was a significant decrease in the following indicators between the basal period and pressure testing and/or induction of ischemia: wrinkled forehead, leg shaking, and self-centered actions. Frowns seemed to be more frequent during the ischemia induction test than during the basal period, but the difference was not significant.
Only the behavioral indicator “frowns” showed differences between the basal and I1 periods (p=0.034), or the I2 (p=0.006), and post-test periods (p=0.010).
The SC group furrowed eyebrows less than the control group during the I1 (p=0.010), I2 (p=0.048), and the post-test periods (p=0.048) relative to the basal period.
Behavioral streams and pain intensity expressionTwenty-nine percent (14/49) of the participants expressed pain of a VAS≥3 during the pain test and 84% (41/49) during the ischemia induction test. The overall behavioral stream of participants with a VAS≥3 was not different (p=0.180) from that of participants who did not express moderate pain. There was no difference between the bodily, facial, or verbal components between those who expressed pain of a VAS≥3 and those who evaluated the pain as a VAS<3.
However, some behavioral indicators differed: those who expressed pain of a VAS≥3 furrowed their brow more frequently (0.28 [0.04; 0.58]/min) than those who did not (0.06 [0.00; 0.16]/min). They also had higher a frequency of moaning (0.08 [0.00; 0.22]/min versus 0.00 [0.00; 0.01]/min).
There was no correlation between the clinical scale (PCS and HAD) and VAS scores or the general behavior stream or specific behavioral components (body, face, voice).
DiscussionIn this study, we compared the behavioral stream of subjects with either SC or MD with that of control subjects free of psychiatric disease. The overall behavioral stream changed during testing, regardless of group affiliation. There was a significant change in the general behavioral streams between the basal period, corresponding to a resting period, without stressful solicitation, and the second part of the pain test consisting of ischemia induction, when the cuff that induced ischemia was deflated. Each of the behavioral components (bodily, facial, vocal) increased between these two periods, with changes in the specific behavioral indicators grimace, massage, moan, and verbalization. The greater occurrence of bodily, facial, and vocal behaviors shows that non-verbal behavior complements the pain expressed by requested verbal evaluation (VAS) during these pain induction tests. There were no differences between the groups based on behavioral profile, suggesting that people with SC or MD behaved similarly during the experiment.
Facial behavior, especially grimaces in all groups and frown in the SC and MD groups, tended to increase. The number of facial behavioral indicators did not increase significantly during the pressure test, but this can be explained by the fact that some participants did not feel moderate or elevated pain (VAS<3). In contrast, they increased significantly during the ischemia induction test, where everybody experienced some pain. This component may be informative as a pain indicator when the pain involves a sufficiently large area or is diffuse. Facial behavioral indicators did not discriminate between subjects with SC or MD and controls. Only the frequency of furrowed eyebrows was found to be linked to pain of VAS>3. The frown corresponds to an indicator identified in the hetero-pain assessment scales for children13,22,23 and the elderly.13 It was not a discriminating indicator between subjects with and without psychiatric disorders during the pressure test, which did not induce pain for all subjects (VAS<3), because the frequency of furrowed eyebrows was similar between the SC and MD groups and controls during this test. However, the SC group furrowed the eyebrows less than the MD group.
This was the most discriminating indicator during the test of ischemia induction. Indeed, the subjects with SC differed from the controls throughout the ischemia test as they furrowed the eyebrows less. Moreover, this indicator appeared to discriminate between the subjects with and without a psychiatric disorder during the late period of the ischemia test, as the subjects with either SC or MD furrowed their eyebrows less than controls. These results are consistent with the decreased facial expression described in the literature for people with SC,24–26 but we did not find any references for people with MD. We did not find any relationship between medication and behavior, consistent with the results of Trémeau et al.,26 which show that the reduction of facial expression of schizophrenic people is due to the disease, and not the medication. They show characteristic facial activities, such as reduced levels of facial expressivity in reaction to emotional stimuli and during social interactions; this reduction can be seen as a behavioral indicator of emotional processing deficits.27,28
There was a significant difference in the body component between the basal and I2 periods, but there were significant changes in the indicators restless legs, self-centered gestures, and massage between the basal period and the various pain tests. The frequency of the indicator restless legs significantly decreased during pressure testing, as did the frequency of self-centered actions during P and I1 for the overall sample. These changes are probably more related to the attention required and the immobility of the upper limb during the pressure or ischemia tests than an indication of pain. We found no link between the body component or indicators and the declaration of pain by a VAS score >3, and thus this decrease is unlikely to reflect the sensation of pain. It can be explained by the attention resulting from the pressure test, during which individuals focus on their arm or algometer and reduce their physical activity. These indicators appear to be of little interest under our experimental conditions, where tests are short and moderately painful, but may be good indicators of sustained attention. The indicator of massage of the affected area has proved interesting for the ischemia test, especially at the end, after removing the cuff, as it increased very significantly for all groups. Indeed, this indicator may be informative if mobility is possible.
There was no significant increase in the frequency of massage during the pressure test, but it may be because the participants had to stay relatively still with their arm under the algometer. However, the frequency of this indicator increased very significantly during the two periods of the ischemia test in which 86% (42/49) of participants felt a pain ≥3 by the VAS. This indicator, which includes massage and movement of the affected area, such as fist clenching, may be relevant for this test because it is consistent with participants seeking to eliminate the tingling/stinging of the ischemic sensation. This indicator is not related to a VAS score ≥3 during testing. It may be a relevant indicator of pain or discomfort in specific situations. It did not distinguish the SC and MD groups.
Finally, the vocal component indicators increased during the tests, particularly moans and verbalization. Such increased verbalization may have been due to most participants experiencing unpleasant sensations or pain during testing, as well as anxiety generated by the experimental situation. Moaning occurred during ischemia induction and was characteristic of those experiencing pain with a VAS>3. Thus, this is a very relevant indicator of pain. These indicators did not discriminate between participants with SC or MD from those free of disease.
We did not find a link between the increase in the frequency of certain indicators and the clinical characteristics of the participants (anxiety, depression, catastrophizing).
There were differences in the variation of HR between groups. MD participants had a smaller change of HR than controls, in agreement with the results of Terhaar et al.29 The differences in the variation of HR between the two groups with psychiatric disorders could presumably relate to drug treatment, but they were related to anxiety or the intensity of pain measured in the study.
The results of an experimental pain test may be influenced by catastrophism or mood (either positively or negatively) and the threat context,30 but we did not observed this here. There were differences in the intensity of depression and general anxiety (measure by HAD) between the various groups, giving them clinical consistency and highlighting the independence of our observations from general depression and anxiety experienced by the participants.
A potential bias in our study was the small size of our sample which did not allow us to carry out more powerful statistical analyzes. However, it was enough to evidence the variations of the naturalistic behavioral indicators along the experimental situation and detect potential variations between groups. These conditions seem to us enough to identify indicators that can be used clinically by non expert health staffs. Additional studies including the use of a fine-grained facial muscle assessment (FACS coding system) would give more fine informations about the presence or absence of specific behavioral signs. However its interest appears limited here because the potential behavioral signs to identify must be recognizable by non expert carers in a clinical setting.
In conclusion, we observed several informative behavioral indicators that reflect pain under our experimental conditions, such as frowning and moaning. We can consider these behaviors as innate or reflexive pain behaviors, present in everyone and probably genetically determined initiated by tissue damage. Other indicators appear to reflect discomfort or inconvenience, such as verbalization and massage of the affected area. Only the frequency of frowning differed according to the psychiatric disorder. The pain behavior based on reflexive, non-verbal behaviors in people with psychiatric disorders is the same as controls, free of disease, in this experimental situation of moderate pain research. No indicator was found to be specific for any specific disease. Most indicators of pain chosen in this study are considered as reflexives behaviors probably influenced by immediate consequences of the experimental situation. The revelation of such an indicator may require a different mode of pain induction (acute, severe, chronic), which we did not use in our study, but which may be potentially observed in routine care. Consequences of some situations of pain more known, frequent or chronic, such as the dentist, a blood test, lumbago can be anticipated and influence people behavior according to their past pain experience. Some behaviors are learned by experience (i.e. self-pity, complaint) and can lead to positive situations (not having to perform certain physical activities, being relieved of social responsibilities, not going to work or school) or positive behavior on the part of others (being cuddled. receiving attention, or even receiving gifts or compensation). There is a learned component of pain that was not focused in this study and that might influence the pain behavior.
Given the existence of many reflexive or innate pain behaviors among the most of people, independently of clinical characteristics, it should be informative to associate to the existing hetero-evaluation scales (such as VAS) some behavioral indicators of simple expression such as frowning or moaning during clinical examination for pain, both in people with or without psychiatric disorders. This study based on the observation of the painful behavior encourage the healthcare team to the recognition of signs of pain among few or non-communicating people, which not necessarily exhibit troubled behavior. This would help to avoid, delays in diagnosis, access to care and treatments for pain. Furthermore, it would avoid denying the pain when orally expressed by psychiatric in-patients. The existence of these behavioral signs indicates without doubt the reality of the pain felt by the patients, when the veracity of the pain verbal expression is questioned, especially in psychiatry.
Compliance with ethical standardsEthical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This open prospective study received legal authorization from the National Agency for the Safety of Medicines and Health Products and the local ethics committee (Clinical trial number NCT01575912).
Informed consent: Informed consent was obtained from all individual participants included in the study.
FundingThis study did not receive funding.
Conflict of interestThe authors declare no have conflict of interest.
We thank Brigitte Plansont for her assistance with the experimental tests and we gratefully acknowledge the contribution of the participants in this study. All authors declare that they have no conflict of interest.
The following are the supplementary data to this article