Although several meta-analyses have suggested the efficacy of non-invasive brain stimulation (NIBS) mainly in prefrontal brain areas to treat mental disorders, no synthetic approach has been performed for other psychiatric disorders rather than depression. The objective is to assess the available evidence of NIBS in the treatment of anxiety disorders.
MethodsAn umbrella review (CRD42021239577) was performed only looking for reviews with meta-analyses of randomized clinical trials using a source strategy MeSH keywords in MEDLINE through Pubmed by two independent researchers. The effects of different methods of NIBS in anxiety disorders were assessed using the PICO strategy. The methodological quality was evaluated using AMSTAR-2 and certainty of evidence using the GRADE-pro framework.
ResultsFrom 136 screening meta-analyses, 16 from 14 studies were included in the final analysis. Generalized Anxiety Disorder (GAD) and Obsessive Compulsive Disorder (OCD) respond best to low frequency repetitive transcranial magnetic stimulation (rTMS), while Posttraumatic Stress Disorder (PTSD) has the largest effect size at high frequency rTMS. Panic Disorder (PD) has no evidence for clinical use of NIBS. There were not identified meta-analyses about other anxiety disorders. In general, the included studies had good methodological quality, but low to moderate evidence for clinical recommendation.
ConclusionAvailable evidence reveals NIBS as an effective and safe approach to treat GAD, PTSD and OCD with low recommendation level to clinical application. A great heterogeneity of studies indicates the necessity to develop new randomized clinical trials applying NIBS to treat those and other mental disorders.
Mental disorders (MD) are a major health concern that represent a significant burden on social participation and are associated with various levels of suffering. The prevalence of MD varies from 7.4 %1 to 22.1 %2; according to the World Health Organization, it is the second leading cause of disability worldwide.3 The moderate efficacy rates and size effects of pharmacological and psychotherapy interventions (first line of treatment for MD)4 stimulated the development of treatment alternatives for these disorders, such as non-invasive brain stimulation (NIBS).
The most common forms of NIBS are transcranial electric stimulation (tES) and repetitive transcranial magnetic stimulation (rTMS). These interventions, based on the modulation of neural circuits, are generally safe and well-tolerated. The most frequently investigated tES technique is transcranial Direct Current Stimulation (tDCS), in which electrical current flows from the positive pole (anode) to the negative pole (cathode), passing through the skin, subcutaneous tissue, skull, and cerebrospinal fluid, and reaching the gray matter that promotes modifications in excitability.5 Furthermore, NIBS is frequently performed through rTMS, which uses powerful, focused magnetic field pulses applied with special coils over the scalp.6 The rTMS magnetic field reaches the gray matter typically without resistance and with little deflection and can elicit action potentials when targeting the underlying cortex. Depending on the protocol used, rTMS can promote changes in cortical excitability, thereby facilitating and/or inhibiting brain areas and networks involved in different cerebral functions.5
NIBS has been extensively investigated as a treatment for MD. Several meta-analyses of randomised clinical trials, mostly for depression and schizophrenia, have been published in the last 30 years.7,8 Although the results are promising, no synthetic approach has been utilised for other psychiatric disorders that have also been associated with maladaptive behaviours, such as anxiety disorders.9 Over 20% of the general population experiences anxiety symptoms.10 According to the ICD-11 (ICD-11: International Classification of Diseases 11th Revision: The Global Standard for Diagnostic Health Information, n.d.), anxiety or fear disturbances include, among others, specific disorders such as generalised anxiety disorder (GAD), panic disorder (PD), agoraphobia (AF), social anxiety disorder (SAD), obsessive-compulsive disorder (OCD), and posttraumatic stress disorder (PTSD). Anxiety also manifests in other types of MD, such as schizophrenia or depression.11
At the brain level, anxiety symptoms cause dysfunction of and connections between some structures such as the amygdala, locus coeruleus, periaqueductal gray matter, anterior cingulate gyrus, insula, and nucleus of the stria terminalis.12 Additionally, the prefrontal cortex and its connected regions are the most important, as their dysfunction may be associated with disbalance of the salience, default mode, and executive networks.13 This region has a special importance in the use of NIBS to control MD because of its superficial anatomical characteristic, the possibility of modulating neuronal circuits, and their respective networks.14
A recent study conducted an important umbrella review (UR) of the use of NIBS to control depression14; it provided the highest quality of evidence in this topic. UR is a qualitative summarization of meta-analyses; therefore, it represents one of the highest levels of evidence synthesis currently available and has been used to expand the knowledge on the application of specific clinical techniques before incorporating them into practice.15–17 There are several meta-analyses on the use of NIBS to control anxiety disorders but no UR; therefore, , we considered it imperative to summarise the best level of evidence in this topic to improve efficacy and security. Hence, this study aimed to assess the available evidence on NIBS for anxiety disorders and to suggest the best protocols.
Material and methodsStudy design and registrationThese URs are part of a broad review produced by the Working Group on scientific evidence for the use of NIBS within the NIBS Brazilian Guidelines Development Group of the NAPeN Network. The protocol for this UR was registered on PROSPERO (CRD42021239577) and published on SSRN (https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3958994) following all recommendations of the PRISMA statement 2020.18
Eligibility criteriaThe study included meta-analyses with a minimum of two randomised controlled trials (RCTs) of the NIBS technique vs. sham groups for the clinical treatment of anxiety disorders. Only studies published in English and with adult participants were included. Studies with duplicate data and surrogate outcomes as well as animal studies were excluded. Furthermore, the most recent update was included in the analyses if there was an update from a previous meta-analysis.
NIBS techniques include transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial random noise stimulation (tRNS), transcranial direct current stimulation (tcDCS), transcutaneous spinal direct current stimulation (tsDCS), transcutaneous vagus nerve stimulation (tVNS), high-definition transcranial direct current stimulation (HD-tDCS), repetitive transcranial magnetic stimulation (rTMS), theta-burst rTMS (TBS), and cerebellar repetitive transcranial magnetic stimulation (crTMS). The eligibility criteria based on the PICOS strategy are summarised in Supplement Box 1.
Information sourcesA systematic search was performed on the PubMed/MEDLINE electronic databases from 8 February 2020 to 20 July 2022 by two independent researchers (LBR and MHC), and articles published in the last 10 years were included. Two independent reviewers (KNS and RFB) extracted data from the selected studies using a standardised extraction procedure. The extracted data included the name of the first author, name of the article, publication year, number of studies, parameters of the NIBS intervention protocol, number of participants in each group (active and sham), outcome measures, number of sessions, adverse events and results, effect size, and their related 95% Confidence Interval. Only the meta-analysis filter was used. All the data were double-checked to ensure accuracy and consistency. Divergence was resolved through consensus.
Search strategyMedical Subject Headings (MeSH) were used for all included MD. All different strategies with the respective numbers of articles found are described in Supplement Box 2.
Selection processEach disorder-specific PICO-question was used to select articles, which are shown in Supplement Box 3.
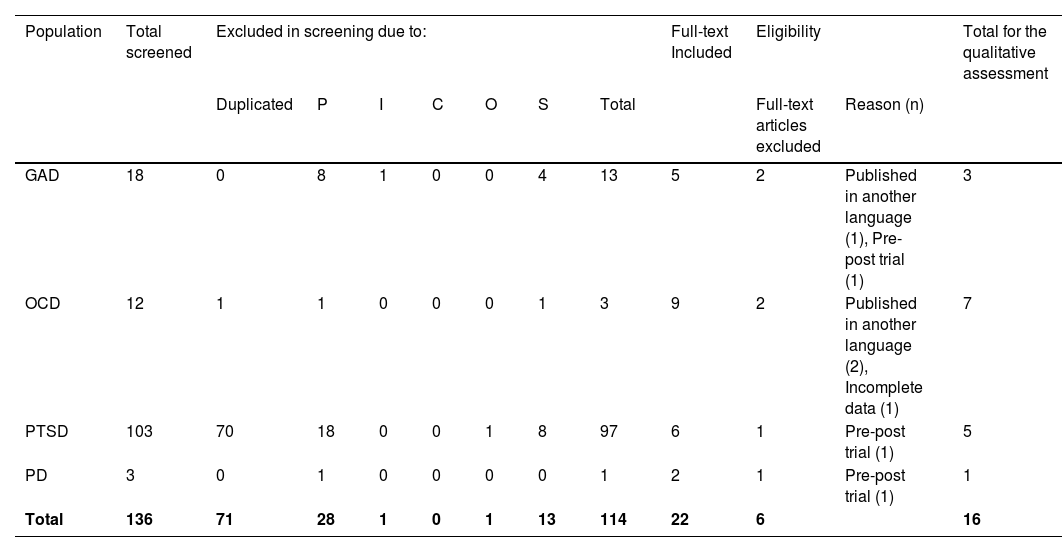
Data collection processFor each PICO question, two independent authors (LBR and MHC) screened the titles and abstracts of the retrieved articles. The full texts of all potential studies were then screened by the same authors based on predefined inclusion and exclusion criteria. Any discrepancies were resolved through consensus or by a third independent author (AFB). The numbers of the screened, excluded, and included studies are reported in Box 1. Of the 14 included meta-analyses, two that presented outcomes for more than one condition were included in this study. Therefore, 16 comparisons were included in this UR.
Data itemsThe extracted data were input into the GRADE system tool (Grading of Recommendations, Assessment, Development and Evaluation Guideline Development Tool, available at www.gradepro.org. The extracted variables were: (1) number of participants (active and control groups), (2) number of responders, (3) number of remitters, and (4) relative (odds ratio [OR], risk ratio, or hazard ratio) or absolute effects. Two authors performed the data extraction process (KNS and RFB) and two authors (LBR and MHC) independently checked the extracted data. Tables were created for the different MD.
Effect measureAll effect size measures (mean difference and odds ratio) were adjusted to the standard mean difference (SMD). For this, we plotted a new meta-analysis with the post-intervention data as the mean and standard deviation (SD) for each study included in the original meta-analysis and performed a new forest plot of SMD. When the means and SDs were not provided, median values were considered to be equal to mean values if data were normally distributed; additionally, interquartile ranges were divided by 1.35 to obtain the SD. If necessary, we also calculated the SD from the confidence interval data provided in the studies, as recommended by Chapter 7 of the Cochrane Handbook. When the study presented the results in only graphs, we extracted the data using WebPlotDigitizer, an extension tool from Google Chrome (available at https://apps.automeris.io/wpd/). All adjusted meta-analyses were analysed using the software RevMan 5 (Cochrane Information Management System). All tests were two-sided, and statistical significance was defined as p=0.05. Homogeneity was evaluated using the heterogeneity test. Meta-analysis was considered homogeneous when the p-value was greater than 0.05, and the heterogeneity index (I2) was up to 30 %. When the heterogeneity was greater than 30 %, a random-effects model was used. When the heterogeneity index was less than or equal to 30%, the fixed-effect model was applied.
Outcome measures to assess relief symptoms were the Hamilton Anxiety Rating Scale (HAM-A) for GAD, self-report for PTSD, the Yellow-Brown Obsessive Compulsive Scale (Y-BOCS) for OCD, and the Panic Disorder Severity Scale (PDSS) for PD. All figures were analysed using Microsoft Excel 365 for Windows.
Study risk of bias assessmentThe quality of all studies was assessed using A Measurement Tool to Assess Systematic Reviews (AMSTAR-2, available online on http://amstar.ca/Amstar-2.php) according to the recommendations of Shea et al.19 This tool uses a checklist of 16 domains to evaluate the quality of RCTs included in systematic reviews.
Certainty assessmentThe quality of each included meta-analysis was assessed considering critical items (2, 4, 7, 9, 11, 13, and 15) and non-critical flaws of the AMSTAR-2 by three researchers (KNS, RFB, and LS). The meta-analyses were classified as ‘high quality’ (none or one non-critical weakness), ‘moderate quality’ (more than one non-critical weakness), ‘low quality’ (one critical flaw with or without non-critical weaknesses), and ‘critically low’ (more than one critical flaw with or without non-critical weaknesses).19 Any discrepancy between authors was resolved through consensus.
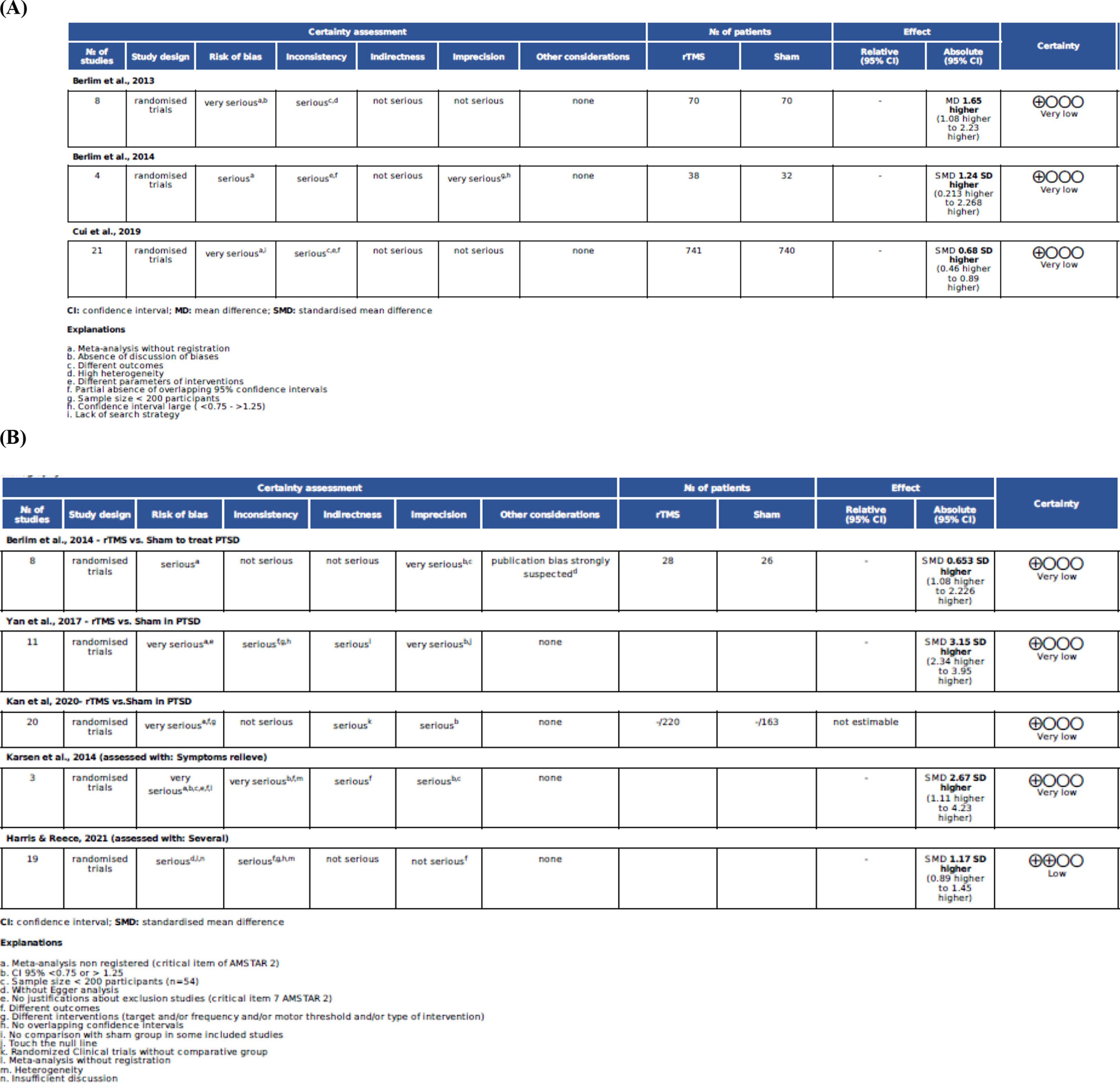
The GRADE tool provides a rating of high, moderate, low, or critically low quality, and a weak or strong recommendation for each outcome. High evidence indicates that future studies are unlikely to change the effect size estimate, moderate means that future RCTs may have an impact on the effect size estimate, low implies high probability that future studies will change the effect size estimate, and critically low implies lack of certainty about the effect size estimate. The GRADEPRO assessments for all the conditions are shown in Figs. 1 and 2.
A qualitative analysis was performed to synthesise the best effect size for each MD and suggest the best protocols.
ResultsA total of 136 meta-analyses were screened, and 14 systematic reviews with 16 meta-analyses were included in this UR, enrolling 3,400 participants (excluding duplicate data) from 112 randomised clinical trials (Table 1). Three meta-analyses were on GAD, five on PTSD, seven on OCD, and one on PD. There were studies that only tested rTMS efficacy, and none tested tDCS for the treatment of selected disorders. However, no studies were found for other anxiety disorders.
Number of screened, excluded and selected studies.
Legend: GAD - generalized anxiety disorder; OCD - obsessive compulsive disorder; PTSD - Post-traumatic stress disorder; PD – Panic Disorders; P – population; I – intervention; C – comparison; O – outcome; S – study design.
The methodological quality of the studies ranged from 7 to 16 points on the AMSTAR-2 scale, with 7, 14, 15, and 16 points, two with 10 and 11 points, and three with 12 and 13 points, respectively. The AMSTAR-2 scores for the high-, moderate-, and low-quality meta-analyses were 5.3 %, 42.1 %, and 52.6%, respectively. No meta-analysis was considered to have a critically low methodological quality. However, in the GRADE-Pro assessment, there were low and critically low evidence levels (Tables 2–5; and Figs. 1 and 2).
Characteristics included systematic reviews about GAD.
Legend: GAD - Generalized Anxiety Disorder; r-DLPFC - right DorsoLateral Prefrontal Cortex; l-DLPFC - left DorsoLateral Prefrontal Cortex; Bi-DLPFC - right and left DorsoLateral Prefrontal Cortex; OFC - Orbito Frontal Cortex; SMA - Supplementary Motor Area; LF - Low Frequency; HF - High Frequency; rTMS - repetitive Transcranial Magnetic Stimulation; HAM-A - Hamilton Scale of Anxiety; SMD = Standard Mean Difference.
Characteristics included systematic reviews about PTSD.
Legend: PTSD - Posttraumatic Stress Disorder; r-DLPFC - right DorsoLateral Prefrontal Cortex; l-DLPFC - left DorsoLateral Prefrontal Cortex; Bi-DLPFC - right and left DorsoLateral Prefrontal Cortex; mPFC - Medium Prefrontal Cortex; M1- Motor Cortex; LF - Low Frequency; HF - High Frequency; rTMS - repetitive Transcranial Magnetic Stimulation; SMD = Standard Mean Difference.
Characteristics included systematic reviews about OCD.
Legend: OCD - Obsessive Compulsive Disorder; r-DLPFC - right DorsoLateral Prefrontal Cortex; l-DLPFC - left DorsoLateral Prefrontal Cortex; Bi-DLPFC - right and left DorsoLateral Prefrontal Cortex; OFC - Orbito Frontal Cortex; SMA - Supplementary Motor Area; LF - Low Frequency; HF - High Frequency; rTMS - repetitive Transcranial Magnetic Stimulation; Y-BOCS - Yellow-Brown Obsessive Compulsive Scale; SMD = Standard Mean Difference.
Characteristics included systematic reviews about PD.
Legend: PD - Panic Disorder; r-DLPFC - right DorsoLateral Prefrontal Cortex; LF - Low Frequency; rTMS - repetitive Transcranial Magnetic Stimulation; HAM-A - Hamilton Scale for Anxiety; SMD = Standard Mean Difference.
Repetitive TMS is a unique neuromodulation modality employed in a variety of forms (high-frequency, low-frequency, accelerated, bilateral, unilateral, and theta-burst). No other NIBS techniques were identified in the selected meta-analyses.
TargetsGenerally, the main target of rTMS for MD is the prefrontal cortex. Specifically, the right and left dorsolateral prefrontal cortices (DLPFC) were employed for interventions for GAD, PTSD, OCD, and PD. Interventions for OCD yielded better results when stimulation was conducted over the supplementary motor area (SMA) or pre-supplementary motor area (pre-SMA), and the left orbitofrontal cortex (l-OFC) was also tested. The right parietal lobe (rPL) was targeted for GAD studies.
Effects of NIBSTables 2–5 and Fig. 2 summarise the results and conclusions of the assessed studies. Most meta-analyses concluded that NIBS led to significant improvements in the psychiatric conditions studied. Despite these positive conclusions, no study revealed the effects of rTMS on PD.20,21
GAD suggested protocolThree systematic reviews with meta-analyses were included, enrolling 21 RCTs, including 1,481 participants, with effect sizes ranging from 0.68 to 2.06 (Table 2). The main stimulated areas were the right DLPFC (n=17 RCTs), followed by the left DLPFC (n=4 RCTs). Among the RCTs included in the meta-analyses, low-frequency rTMS was used in most studies (n=20), and high-frequency rTMS (10–15 Hz) was used in only one study. The amplitude varied from 80 % to 110 % of the resting motor threshold (RMT), with 90 % being more frequent (n=9). The number of pulses varied from 500 to 2,400, and the number of sessions ranged from 10 to 30. The best results were observed for 10 sessions of low-frequency rTMS (1 Hz) on the right DLPFC at 90 % RMT and 2,400 pulses. Adverse effects such as headache, neck pain, scalp pain, tingling, sleepiness, facial twitch, and impaired cognition were reported in the included studies.
PTSD suggested protocolFive systematic reviews with meta-analyses were included, enrolling 26 RCTs, including 677 participants, and presenting effect sizes ranging from 0.98 to 3.15 (Table 3). They assessed rTMS applied unilaterally (n=16 RCTs) or bilaterally (n=4 RCTs) over the DLPFC, in the medial PFC (n=1 RCT), or in the primary motor area (n=1 RCT), or did not present details about the location of stimulation (n=2 RCTs). They used frequencies varying from 1 to 20 Hz, intensities varying from 80 % to 120 % of the RMT, in 10 to 36 sessions, and using a total of 7,500 to 60,000 pulses. The best results were found using an excitatory protocol (10 Hz) over the r-DLPFC, with 100 % of the RMT and 18,000 pulses for six weeks as an additional therapy.9,22,23 Minimal adverse effects were observed, including headaches (n=5/563 individuals) and dizziness (n=2/563 individuals). However, using 20 Hz, a single generalised tonic-clonic seizure was reported in two RCTs.24,25 The adverse effects reported in the majority of included studies were mild headache and intrusive thoughts.
OCD suggested protocolSeven systematic reviews with meta-analyses were included, enrolling 28 RCTs, including 951 participants, with effect sizes ranging from 0.59 to 3.89 (Table 4). They assessed rTMS applied bilaterally over the right DLPFC (n = 4), left DLPFC (n = 4), right DLPFC (n = 7), and pre-SMA as an additional stimulus (n = 2), and only pre-SMA (n = 3), pre-SMA (n = 5), and left or right OFC (n = 2) targets. The intensity varied from 80 % to 120 % MT from two to six weeks. The best results point to inhibitory protocols applying 1 Hz, with 100 % MT, over the right, left, or both DLPFC with or without SMA over 20 sessions spread over five times a week of rTMS. No meta-analysis used tDCS as a NIBS to treat OCD. None of the included studies reported any adverse effects.
PD suggested protocolA single meta-analysis was performed to assess rTMS in PD [20], enrolling 52 participants, without differences between the groups (Table 5). The standardised mean difference (SMD) was 0.08 (95 % CI = -0.44-0.60) between groups. This Cochrane meta-analysis showed that the included RCTs had small sample sizes, large confidence intervals, and high heterogeneity. Therefore, we did not present the suggested protocol. The included studies did not report any adverse effects.
The summarised data of the best effect sizes in this UR for all analysed health conditions are presented in Table 6.
Best effect sizes in NIBS in the summarized meta-analyses for GAD, PTSD and OCD.
Legend: GAD - Generalized Anxiety Disorder; PTSD - Post-Traumatic Stress Disorder; OCD - Obsessive Compulsive Disorder; r-DLPFC - right DorsoLateral Prefrontal Cortex; SMA - Supplementary Motor Area.
This umbrella review summarises the existing evidence on the use of NIBS in the treatment of some anxiety disorders, including GAD, PTSD, OCD, and PD. To the best of our knowledge, our study is the first UR on this topic. Among all NIBS strategies, rTMS was the only technique assessed in the available meta-analyses for controlling anxiety symptoms. Interventions were tested in 112 RCTs summarised in 14 systematic reviews of RCT and 16 meta-analyses, as two articles included two meta-analyses. In those studies, a third of the patients were non-responders to pharmacological interventions, and 49 % of those patients achieved remission of symptoms using NIBS, which is in accordance with a previous study.26
The number and quality of meta-analyses in this area have improved significantly in the last few years.8 However, most of the included meta-analyses were classified as low quality, especially due to the imprecision of results and high risk of bias. Its scenery points to the necessity of developing several new and qualified clinical trials in the theme of testing NIBS protocols for MD.
Effects of NIBS on the treatment of GADAlthough pharmacotherapy and psychotherapy can be considered first-line options in the treatment of GAD symptoms,27 non-adherence to treatment and/or difficulty in maintaining drug therapy may be important factors for worsening the condition. The included meta-analyses demonstrated symptom reduction without significant heterogeneity between studies and a large effect size applying rTMS to treat GAD.22,28–31
In general, the primary outcomes can be assessed using the Hamilton Anxiety Scale (HAM-A); the composition of drug therapy along with the use of neuromodulation decreased anxiety symptoms in GAD.29 Treatment with active rTMS has been considered a safe and well-tolerated treatment method31 Despite meta-analyses suggesting good results of the application of high or low frequency over the r-DLPFC,9 the best results were achieved using 1 Hz over the r-DLPFC. These results were also observed by Fitzsimmons et al.32 The number of sessions and intensity must be considered on a case-by-case basis considering co-morbidities, available resources, cost-benefits, and disponible time. Although accelerated protocols associated with psychotherapy techniques can help people with GAD, they need to be tested in well-delineated RCTs in the future.
Effects of NIBS on the treatment of PTSDIn PTSD treatment, there is evidence for decreased anxiety symptoms with rTMS applied to both the right and left DLPFC.22 The primary outcome was assessed using self-report symptoms and/or HAM-A despite a specific instrument to assess PTSD—the Posttraumatic Cognitions Inventory (PTCI). Diverse and heterogeneous mechanisms of action and the ability to act broadly or very locally may enable brain stimulation devices to address core PTSD symptoms in more targeted ways.33 The main stimulated area is the DLPFC (right, left, or both sides) and medium PFC. A single RCT applied rTMS in the motor area with moderate effect size. In all five selected meta-analyses, the active group was consistently superior to the sham group for all outcomes.
Comparing low and high frequency, 10 Hz was better than 1 Hz to treat PTSD.13 Two RCTs applied 20 Hz and observed the adverse effects.25,34 Therefore, to ensure patient safety and comfort, caution is necessary with parameters of rTMS to treat PTSD being recommended to apply 10 Hz. The use of rTMS evolved between 2014 and 2020, increasing the level of evidence for its use from low to moderate.8
Effects of NIBS on the treatment of OCDOCD studies frequently included participants who were unresponsive to drugs and behavioural therapy35; additionally, they showed larger effect sizes than other anxiety disorders, although with high heterogeneity. The Y-BOCS is the gold standard for assessing OCD and was used in all the selected studies. Both obsessive and compulsive behaviours decreased with the use of rTMS as an additional therapy to pharmacological and psychotherapeutic therapies.
Regarding the best target in the treatment of OCD, the left, right, and/or bilateral DLPFC promoted significant improvements compared with the effects of sham treatments, with the highest effect size on the bi-DLPFC, followed by the mPFC and right DLPFC,36 using 100 % motor threshold and 1 Hz of frequency.37 Similar results were observed in a meta-analysis that included the SMA.38 In contrast, Fitzsimmons et al.32 observed that the best effect sizes were with DLPFC stimulation in comparison with OFC and mPFC, primarily on the right side. However, a single meta-analysis suggests that low-frequency rTMS is more effective than high-frequency rTMS in SMA after 12 weeks compared to high-frequency rTMS in the DLPFC.23 It is possible that all protocols reduced front-striatal hyperconnectivity, as demonstrated by one study,39 or promoted the normalisation of hyperactive orbitofrontal-striatal circuits enrolled in cognitive and emotional processes, which can explain these paradoxical results.40,41
The optimal parameters for the treatment of OCD have also been investigated in some studies. A recent meta-analysis of NIBS for OCD found the best effect size applying 1 Hz over the bilateral-DLPFC,36 although another showed the best effect size applying 1 Hz over the right DLPFC.32 When considering both frequency and tolerability in 22 RCTS, Liang et al.42 showed the best effect sizes with 1 Hz over the r-DLPFC, 1 Hz over the SMA, and 10 Hz over the ACC/mPFC. While these discrepancies are difficult to explain, it is possible that the ‘top-down’ effect of rTMS43 is more global than local,44 and is the most important mechanism in the control of MD, irrespective of the target to be stimulated.
Effects of NIBS on the treatment of PDA Cochrane review demonstrated a small number of RCTs testing the efficacy of rTMS in the treatment of PD, with small sample sizes and insufficient data to draw any conclusions.20 Eight years after this publication, the efficacy of NIBS in treating PD remains inconsistent. The findings showed small effect sizes, regardless of underlying comorbidities or NIBS parameters.21 Unfortunately, we could not include this recent meta-analysis because it included pre-post trials, which was one of our exclusion criteria.
Adverse effects of NIBS on the treatment of anxiety disordersMost meta-analyses did not present a detailed report or OR analysis of the occurrence of adverse effects. However, some severe events, such as impairments in cognition and intrusive thoughts, were described.
Clinical applicationDespite decades of research on the treatment of humour and other MD, these disorders remain a major social health problem. The unfamiliarity of mental health professionals with NIBS has contributed to the historical marginalisation of brain stimulation in health care.45 However, incorporation of NIBS in the treatment of MD can potentiate the results of pharmacological and psychotherapeutic techniques, being supportive and non-competitive resources or helping non-responder cases. Summarising evidence about the efficacy and security of NIBS can support clinicians and health managers in incorporating this technology into treatment options.
Although the classification of the included meta-analyses on GRADE-Pro was ‘very low’, the RCTs indicate a sound perspective to treat PTSD with rTMS but with low level of evidence regarding the clinical practice. PTSD and OCD have the best responses to rTMS, with PTSD using excitatory protocols (10 Hz) over the right DLPFC and OCD using excitatory protocols (10 Hz) over the right DLPFC associated with inhibitory protocols (1HZ) over the pre-SMA. An important limitation is that both conditions can present varying levels of comorbid depression and anxiety, which may influence the responses to rTMS. People with high levels of anxiety respond better to inhibitory protocols over the right DLPFC protocol,9 while those with depressive conditions respond better to excitatory protocol on the left DLPFC.14 Recent studies have revealed interhemispheric asymmetry of cortical excitability in mood disorders associated with dysregulation of membrane excitability in pyramidal neurons and of gamma-aminobutyric acid (GABA) and glutamate neurotransmission.46 Hence, when prescribing a treatment protocol, it is necessary to consider the patients’ comorbidities and individual profiles.
GAD, PTSD, OCD, and PD share the basic feature of anxiety, which explains their common response to pharmacological and psychological interventions similar to NIBS in different protocols.47 rTMS neuromodulation techniques have proven effective in reducing symptoms. According to our study, there is moderate evidence for clinical use of rTMS. The use of NIBS for the treatment of GAD, PTSD, OCD, and PD is promising; future investigations should confirm this possibility and include rTMS as a possible first-, second-, or third-line treatment for anxiety disturbances.29 However, there is no single protocol for these disorders. Additionally, to translate the research findings into clinical practice, it is necessary to carefully analyse each patient's condition.
Our search was performed only in the PubMed database, and we included meta-analyses only in the English language, which constitute the limitations of this study. Furthermore, we calculated SMD based on MD or OR, which may have increased the risk of bias. We prefer to use this analysis to summarise the results of different meta-analyses in a more standardised manner. Another limitation of this study is that most studies used rTMS adjunct to other treatments to assess incremental advantage and obscure the effects of the underlying primary treatment. The major limitations in the meta-analyses to produce this classification were the absence of registration, inconsistency due to high heterogeneity between the included studies, small sample sizes in the RCTs, and the consequent large confidence intervals. Heterogeneity of study protocols because of small sample size, sample characteristics like the presence of comorbidities, pharmacological and psychotherapeutic interventions, and diversity in rTMS parameters constitute a difficulty in the assessment of evidence level. To extrapolate the data presented in this UR to clinical practice, caution is necessary because various mental health conditions, presence of comorbidities, economic aspects, and sociocultural determinants of health can generate different results.
ConclusionThe results of this UR, which included 16 meta-analyses evaluating the efficacy of rTMS in controlling anxiety in MD (GAD, PTSD, OCD, and PD). The results were robust regarding the effect sizes; however, the methodological quality of the studies showed low-to-moderate levels of evidence for GAD, PTSD, and OCD, and no evidence for PD. The great heterogeneity of studies indicates the necessity of developing new randomised clinical trials that apply NIBS to other therapeutic protocols and with larger sample sizes to treat these mental disorders.
Ethical considerationThe authors declare that they have paid careful attention to how the perspectives of the authors and research participants of original studies are represented—in a way that makes the missing perspectives visible. The domain of applicability of systematic reviews was scrutinised to deter the unintended extrapolation of review findings to contexts where they were not applicable. Furthermore, the authors reflected on how biases could influence our findings to guarantee internal and external validity and reliability. Every effort was made to ensure maximum transparency and reproducibility of the findings.
FundingAFB is supported by CNPq/Brazil (Grant 314149/2018-0); ARB receives scholarships and support from FAPESP, the Brazilian National Council of Scientific Development (CNPq-1B), University of São Paulo Medical School (FMUSP), the UK Academy of Medical Sciences (Newton Advanced Fellowship), and the International Health Cohort Consortium (IHCC); KMS is supported by CNPq/Brazil (Grant No. 31 1224/2019-9); KNS is supported by FUNADESP/Brazil (Grant No. 60-123/2022). This research was not supported by any funding sources.
Author contributionsAll authors substantially contributed to the conception or design of this study, or acquisition or analysis or interpretation of data. All authors revised it critically, having important intellectual content; all authors revised the final version submitted for publication; all authors agreed to be accountable for all aspects of the work to ensure that questions related to the accuracy or integrity regarding any part of the study are appropriately investigated and resolved. KNS, CT, AFB, and KMS conceived and promoted the training of the research team and supervised the methodology. LBR, MN, MHFC, and LAS were responsible for data collection. KNS, RFB, and LS performed the data analysis. KNS, RFB, and LBS wrote the manuscript. KNS, AFB, and ARB reviewed the final manuscript.
The authors would like to thank Andrea Lemos, Lázaro Juliano, Adriana Baltar, Maíra Souza, Rodrigo Brito, Deborah Marques, and Gabriel Barreto for their valuable help and expertise on methodological analysis.