Cancer of the uterine cervix affects nearly 500 000 women worldwide;1 Mexico has one of the highest incidence (40 × 100 000 females)2 and mortality rates (17 × 100 000 inhabitants) for this type of cancer.3 It has been demonstrated that more than 90% of cervical carcinomas are associated with persistent infections of oncogenic genotypes of the human papillomavirus (HPV), mainly types 16 and 18.4
Despite that intensive early-detection campaigns have been conducted in Mexico for more than 30 years,3 it has not been possible to diminish the incidence or mortality of this disease since the sensitivity of conventional cytology is low (50-60%).2
In order to increase the sensitivity of screening methods, new ancillary techniques have been implemented at a national level in Mexico. Among these are liquid-based cytology and molecular biology tests.
Hybrid capture test (HC2), which detects 13 of the most frequent genotypes of the high-risk HPV virus, is the molecular biology test accepted by the Federal Drug Administration (FDA) since the year 2003.5,6 This study has the following indications: a) patients with a cytological diagnosis of atypical squamous cells (ASC); b) post-treatment follow-up; and c) as a support of cytological cervical screening in women over 30 years of age.2
Several studies have also included polymerase chain reaction (PCR) HPV diagnosis as a reinforcement of HC2 test, due to its high sensitivity.2,5,7,8
The first indication to perform these tests is a cytological interpretation of ASC. This term was introduced by the Bethesda System of Reporting Cervical Cytology in 19889 and modified in 2001.10
In the U.S.A. this diagnosis is reported in nearly two million women annually,5 and in the National Cancer Institute in Mexico City, in 1-1.5% of reviewed cytologies.11 The term ASC refers to borderline lesions that surpass the reactive processes but that do not bring together the requirements for diagnosis of a low-grade intraepithelial lesion that requires strict follow-up.
Cytomorphological criteria for diagnosing ASC are well established, but are subjective.10 Therefore, an overdiagnosis of this entity predominates, with the consequent increase in colposcopy studies, and unnecessary treatments with excessive costs.12
The objective of the present work was to evaluate whether cytomorphological criteria used in diagnosis of ASC at the National Cancer Institute of Mexico correlate with the presence of high-risk HPV types, justifying thus the performance of molecular biology tests (HC2 and/or PCR) for follow-up of only positive cases.
¿METHODS
This study included 100 patients selected with a cytological report of ASC. This diagnosis was performed with conventional cytology applied to patients assisting to theGynecological Department of the National Cancer Institute of Mexico from 2007-2008. Patients signed a letter of informed consent. Exclusion criteria included the following: refusal to participate in the study and insufficient sample amount for molecular diagnosis. A prospective, observational, and transversal study was conducted. We considered the following as risk factors: age; number of sexual partners, and age of first sexual intercourse (FSI).
Patients diagnosed with ASC were required for a new sample for molecular biology studies: HC2, and PCR.
Initial conventional cytology was carried out with an Ayre spatula and cervical brush from exocervix and transformation zone. The sample was fixed with 96% alcohol and stained according to the Papanicolaou technique. For HC2 and PCR tests, samples were taken with a cervical brush and fixed in STM Digene Liquid and 1 ml Lysis buffer (Tris-HCl 10 mmol/L pH 8.0, EDTA 0.1 mol/L pH 8-0, SDS 0.5%, Proteinase K 200 μg/mL, RNase A 20 μg/mL), respectively.
Initial ASC diagnosis was made during routine laboratory work with conventional cytology samples stained with the Papanicolaou technique, screened by a senior cytotechnologist, and reviewed by an experienced cytopathologist.
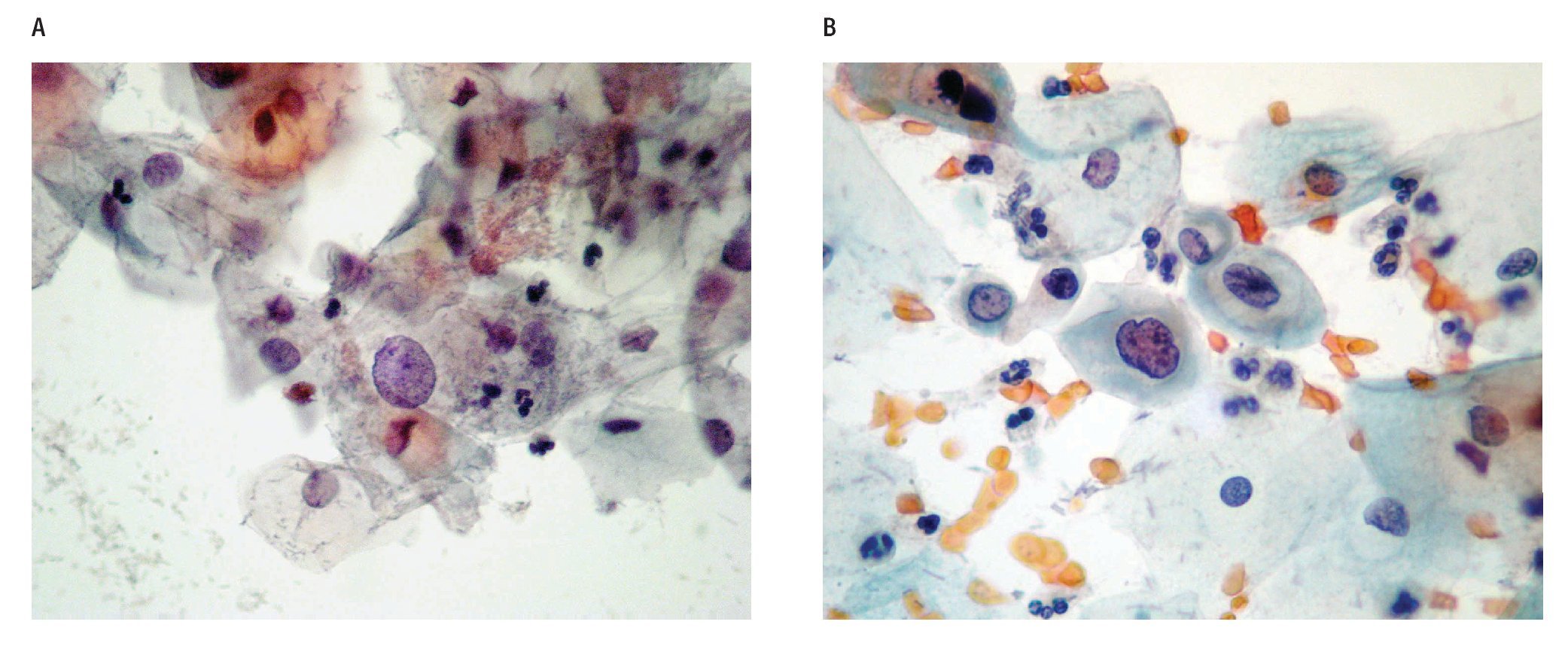
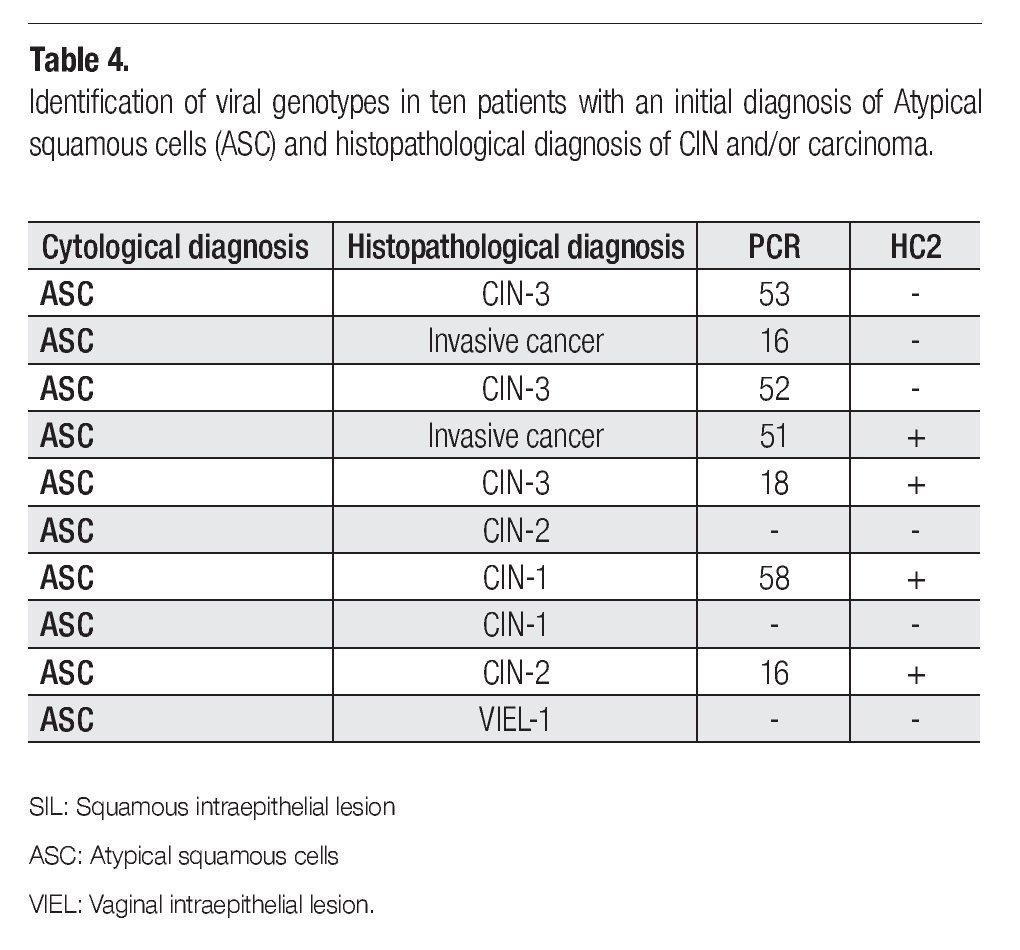
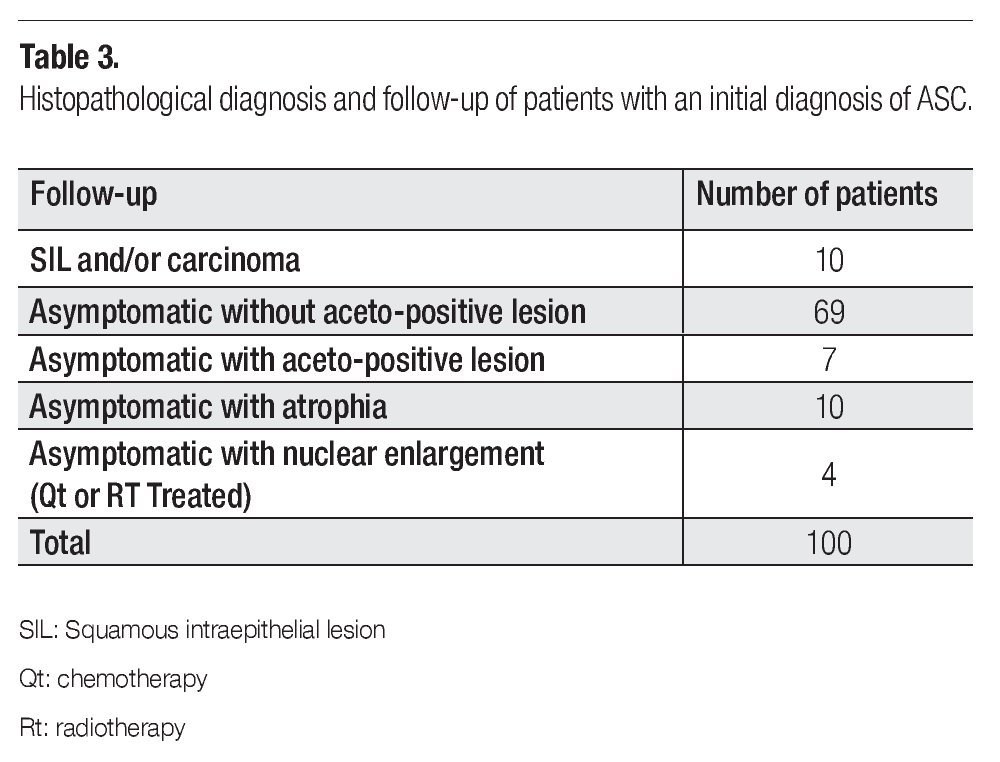
The cytological diagnostic criteria were uniform according to the Bethesda System of Reporting Cervical Cytology 200110 guidelines, which state that an ASC should show an increase in nuclear size of 2.5-3 times related to the nucleus of normal intermediate cells, slight irregularity in the nuclear contour and a slight change in the chromatin pattern (Figures 1a, b).
Figure 1. ASC diagnosis: a) Papanicolaou staining: altered intermediate cell with nuclear growth 2.5-3 times the size of the nucleus of a normal intermediate cell (400x). b) Papanicolaou staining: altered deep cell, with slight growth and nuclear irregularity, slight hyperchromatism (400x).
HPV DNA detection by the HC2 method (Digene Co., Gaithersburg, MD, USA) comprises a single stranded DNA hybridized with RNA probes directed to high- and intermediate-risk viruses (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), and a chemioluminiscent detection of the signal. PCR is an enzymatic amplification of DNA that allows the detection of very low levels of DNA copies through an exponential amplification of a selected region. For HPV detection samples were digested with 1 mL of lysis buffer at 55 ºC for 3 h. DNA was extracted with phenol/chloroform precipitations as described by Sambrook et al.13 As extended DNA quality control DNA was amplified for ß-globin gene (PCO4/ GH2O) under conditions described by Resnick et al.14 Samples were later submitted to HPV amplification with three sets of the following universal primers recognizing distinct size fragments of L1 gene: L1C1/L1C2, MY09/ MY11, and GP5/GP6.15,16 DNA extracted from CaSkiand Hela-HPV positive cell lines were used as positive controls. Each amplification reaction was carried out in a 20-μl volume containing 2 mM dNTPs, 0.5 U de Amplitaq Gold DNA polymerase, 1.5-3.0 mM of MgCl2, buffer 10X, 10 pM of each oligonucleotide, and water. Temperatures of denaturation, alignment, and extension depended on the oligonucleotide set employed. HPV PCR products were electrophoresed in a 1.2%agarose gel and visualized by ethidium bromide staining.HPV typing was carried out by direct sequencing ofPCR products by means of the BigDyeTM Terminatorv3.1 Cycle Sequencing Kit (Applied Biosystems). Theresulting sequences were analyzed in BLAST data bankfor comparison with known HPV sequences.
Kappa agreement between the two tests (HC2/ PCR) was calculated.17 Cytological diagnosis data (ASC) and positivity for HC2 and PCR tests were correlated with clinicopathological variables analyzed. Patient follow-up was carried out at least for one year.
¿ RESULTS
Age of the 100 patients included in this study ranged between 18 to 80 years, with a mean of 43 years and a median of 46 years. Descriptive values of the studied variables according to its relation with presence or absence of HPV, were analyzed. Risk factors for developing squamous intraepithelial lesions (SIL) and/or carcinoma considered in this study were the following: age, number of sexual partners, and age at first sexual intercourse. Forty one patients (41%) declared one sexual partner. First sexual intercourse was declared in a range between 11-49 years, with an average age of 27 years and a median age of 20 years. Variables were analyzed in order to find any association with HPV positivity. Neither of the variables showed any significant association, including declared number of sexual partners.
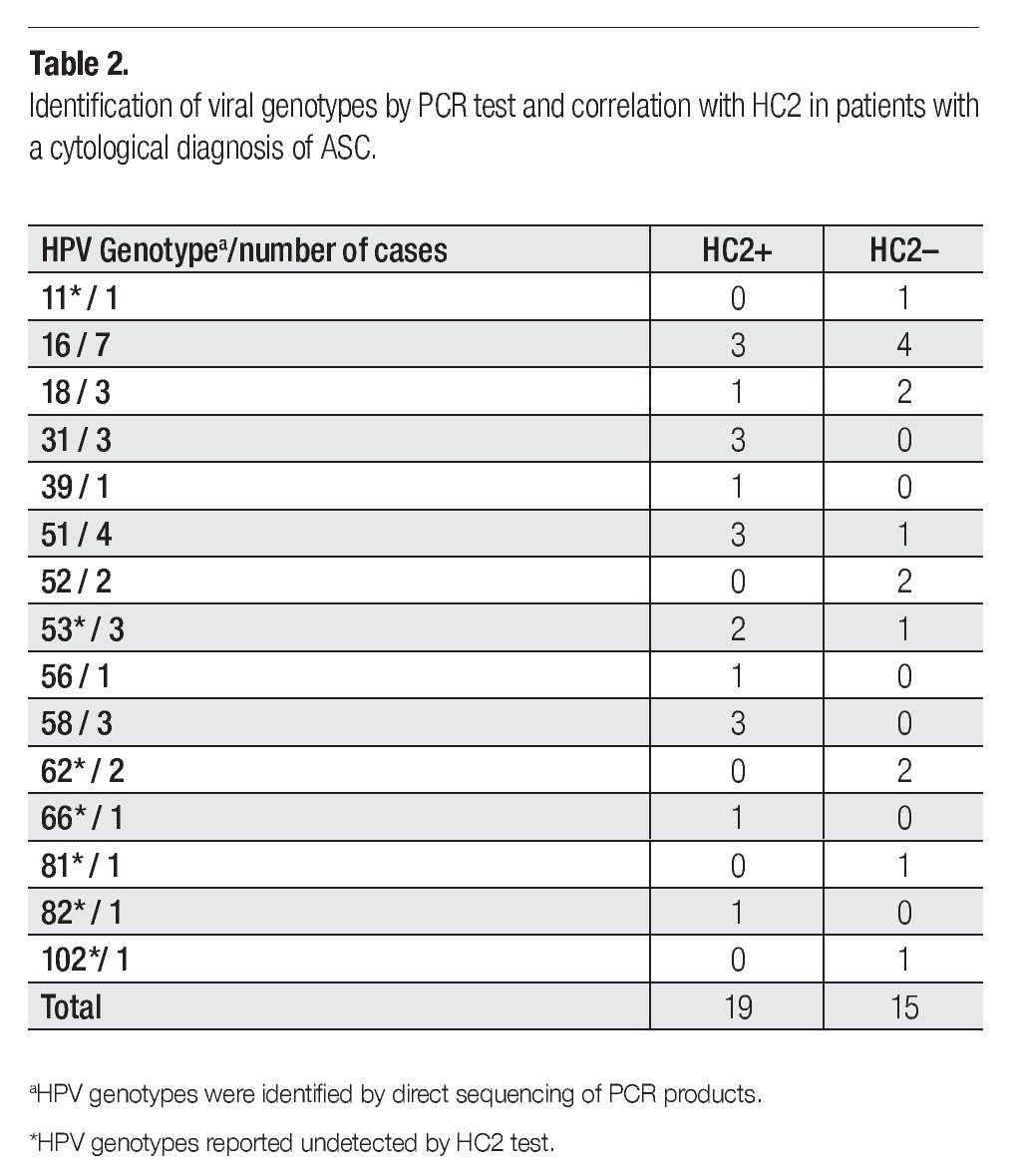
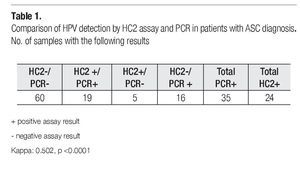
Table 1 shows a comparative analysis of HC2 and PCR for HPV DNA detection. A total of 40% of the patients were HPV positive by any assay. Particularly, the PCR assay detected 35 HPV positive cases, while HC2 test detected 24 positive cases. 19% of the samples resulted HPV positive for both tests, as 60% were negative for both test. Meanwhile, 21% had a discordant result (16 % were only positive for PCR amplification; and 5% were only positive by HC2). Kappa value was 0.502 (IC 95% 0.313-0.691) with 50.2% of agreement between the two methods, which was greater than that expected by chance; hence the interpretation was a moderate agreement, and the significance level was p <0 0001 with 95 confidence intervals of 0 313-0 691
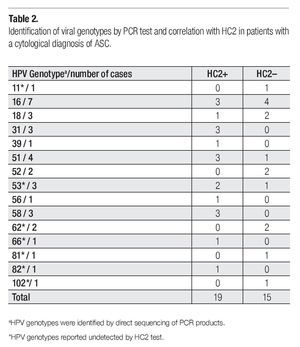
Viral genotypes were identified by direct sequencing of PCR products. Correlations with HC2 findings are shown in Table 2. Cases with viral genotypes 11, 62, 81, and 102 were found negative in CH2 test, which is explained by the fact that these viral types are not included among the 13 genotypes detected by this method. Nevertheless, two cases positive to HPV53, one to HPV66, and one to HPV82, tested positive also for CH2 even though these viral types are neither included in the geno-types detected by HC2 test.
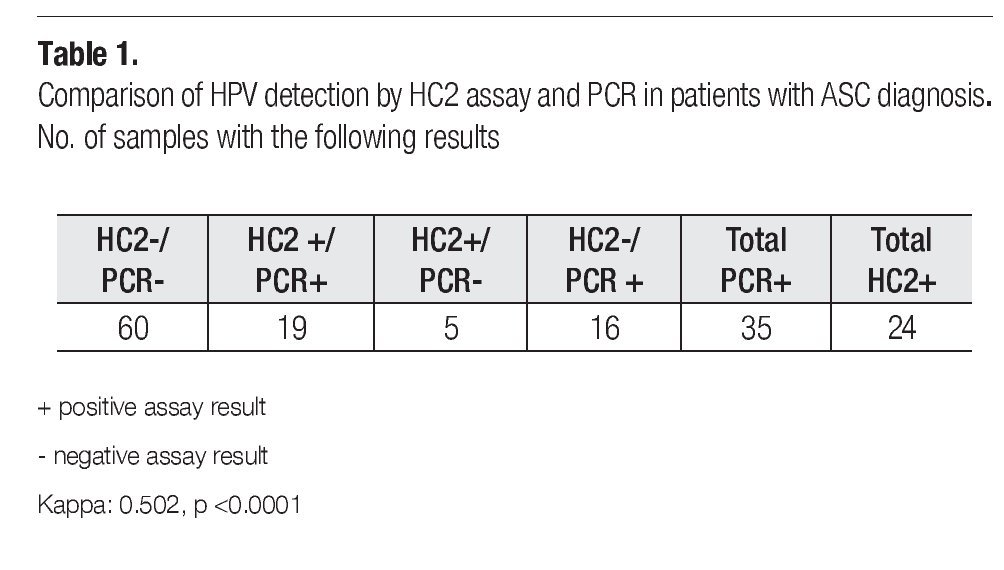
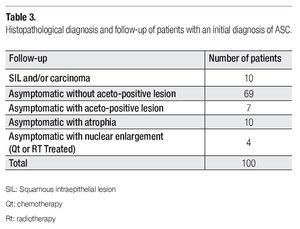
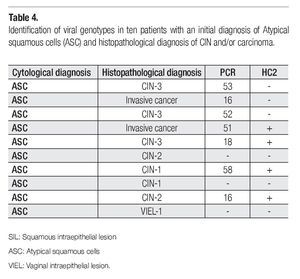
Clinical or histopathological data of patients with one-year follow-up is shown in Table 3. Most of the patients continued asymptomatic (90/100). Seven of them presented aceto-positive colposcopical lesions; ten, atrophic epithelia; and 4 patients presented nuclear enlargement due to chemo (QT)- or radiotherapy (Rt). The most important observation was that obtained for 10 cases that developed SIL (squamous intraepithelial lesion) or cervical carcinoma in one-year follow-up. In Table 4 are disclosed the histopathological diagnosis of these ten cases and their correlation with HPV results. Seven cases resulted positive for high-risk HPV types, as classified by de Villiers et al,18 (HPV types 53, 16, 52, 51, 18, and 58), from which only four were detected by HC2. However, three precursor lesions: one cervical intraepithelial lesion (CIN-1), one vaginal intraepithelial lesion (VIEL-1); and one CIN-2 resulted HPV negative by both tests.
¿ DISCUSSION
In cervical cytology screening, atypical squamous cells of undetermined significance (ASC-US) is an equivocal category assigned to specimens with morphologic changes suggestive of cervical intraepithelial neoplasia but which may also represent non-neoplastic conditions of various causes.5,9,10 Therefore, it is a borderline lesion that requires strict follow-up. Cytological diagnostic parameters for this entity are subjective and if interpretation is incorrect, it can give rise to an over or under diagnosis with the consequent expensive follow-up and patient miss-treatment. In the USA, two million women are diagnosed with ASC annually.5 The cost of follow-up and overtreatment of these patients is very high. Kulansingam and colleagues12 report that performing a colposcopy in every women with a cytological diagnosis of ASC is more expensive than performing it exclusively in women with cytological diagnosis of ASC and HPVDNA positive test ($20 370 vs. $3117dollars (USD) / HSIL diagnosed case).
Since quality assurance for the interpretation of ASC is important, international guidelines recommend that ASC diagnosis should correspond to no more than 5% of the laboratory cytological material, or that the ASC diagnosed cases should be no more than 2-3 times the number of SIL cases.10 In such sense, is relevant to mention that at the cytopathology laboratory of the National Cancer Institute of Mexico, one to 1.5% of the studies (with a mean of 15 cases per month) are reported as ASC.11 Nevertheless, it is clear that the ASC/SIL ratio as a measure of quality assurance may be an imperfect tool due to the subjective parameters used in the cytological diagnosis of ASC. Therefore, as other authors have proposed,12,19 HPV DNA testing may be helpful to improve follow-up triage. Different authors cite that 34 to 50% of ASC diagnosed cases will be positive for high-risk HPV DNA.19,20
Lörincz et al5 mention the benefits of DNA HPV tests, since HC2 tested positive in 100% of HGSIL, compared with 84% of HGSIL detection by means of liquid-based cytology tests and 58% of conventional cytology tests. More recently, as reported in the ASCUS/Low-grade intraepithelial lesions study (ALTS),19 HC2 sensitivity was 96.3% compared with 85.3% for liquid-based cytology.
Zahn et al6 consider that the combination of cervical cytology and DNA HPV tests is more sensitive for detecting HGSIL than any of the methods separately. These tests may reduce the diagnosis of invasive carcinomas in 90-92%, compared with 89% of conventional cytology, and may increase the negative predictive value up to 99-100%,2 which would justify the extension of the screening interval to approximately five years.
It is important to be aware that HPV DNA results can differ depending on the techniques used. In this study we considered necessary to analyze if the morphological changes found in ASC diagnosed cases were associated to an HPV DNA positive test. We were also interested in comparing both tests, HC2 and PCR, for HPV detection in those samples.
Sixty four percent of cases in the Guo series8 and 40% in our study were positive for one or both tests performed (HC2 and PCR). Meanwhile, 60% of our cases resulted HPV DNA negative by the two methods employed. When comparing results obtained by both tests, we found a 21% of disagreement between HC2 and PCR tests. These data are similar to that shown by Cañadas et al,7 who also compare both tests obtaining a 16% of discordant results.
According to Meijer et al,4 patients who test negative for HPV DNA have a minimum risk of developing SIL and/or carcinoma (40-50% lesser risk in 5 years), suggesting the HC2 performance to conduct follow-up studies exclusively in patients positive for high-risk viral genotypes.
We found that HC2 technique detects a less number of positive cases (24 HPV DNA positive women) than those detected with PCR (35 HPV-positive women), as reported by Cañadas et al.7 This discrepancy can be attributed to a higher sensitivity of the PCR method, which can detect a very low number of viral copies. However, according to several authors, a very low number of copies may have no risk of progression.4
The five PCR- and HC2+ cases can be explained by cross-reactions with other genotypes, generally of low risk, which are not efficiently detected by the PCR oligonucleotides. Otherwise, failures in PCR amplifications due to deletions or sequence intra-type variations can also affect the PCR result, affording a false-negative.7
Is worth to mention that HPV-16 was the most prevalent genotype found in our study (20.6% of the HPV DNA positive cases), followed by HPV-51 (11.7%), and the third place with similar proportions were for HPV-18, -31, -53, and 58. Uncommon types as HPV-102, -82 and -81, were also found.
The 60% of HPV DNA negative may be over diagnosed ASC cases, as suggested by Meijer.4 Nevertheless, three precursor lesions detected at the one year follow-up, resulted negative for HPV by both tests. Two of the latter cases corresponded to low-grade SIL (CIN-1 and VIEL-1), which may have a low risk of progression.21 However, the third case was a high-grade SIL (CIN-2), which may mean a false negative of both HPV detection methods. When trying to explain the possible causes of ASC over diagnosis, we found that 10 women were in the fifth decade of life, with atrophic epithelia. These atrophic morphological changes may be miss-interpreted as ASC because cervical atrophy is frequently accompanied by exaggerated nuclear growths. Four percent of patients had been previously treated with radiotherapy (Rt) and/or chemotherapy (Qt), which also have been associated with nuclear growth. However, the majority of miss-diagnosed cases (69 patients) may correspond to borderline inflammatory lesions that possibly may need no follow-up.
Follow-up of HPV DNA positive patients is necessary. Different authors report that during 2 years, 60% of patients become HPV negative during this period and have no risk of developing SIL and/or carcinoma. Whereas according to different series, 10-36% of patients with a cytological diagnosis of ASC and positive for high-risk virus develop high grade SIL.14,16
In conclusion, the percentage of diagnosis of ASC at the Department of Cytopathology at the National Cancer Institute of Mexico remains within the parameters accepted by the international literature.9 Nevertheless, only 40% of the cases included in this study were positive to HPV DNA, possibly meaning an over diagnosis of ASC in the majority of HPV negative cases. Seven of ten cases that in a one-year follow-up progressed to SIL or cervical cancer, were positive to high-risk HPV types. These results justify the performance of molecular biology tests, preferably both HC2 and PCR, in ASC diagnosed patients, to strictly follow-up those with HPV DNA positive results.
¿ ACKNOWLEDGEMENTS
This work was partially supported by CONACyT grant 69875. We thank Dr. Sergio Ponce-de-Leon for statistical assistance.
Correspondence: Dra. Rita Sotelo Regil Hallmann.
Av. San Fernando N° 22. Col. Sección XVI, Tlalpan 14080 Mexico, D.F. Mexico.
Telephone/fax: 5628 0468.
E-mail: rsotelo24@hotmail.com