¿ INTRODUCTION
About two thirds of breast cancer patients do express hormone receptors and probably are to some degree dependent on estrogen and progesterone for development, growth and progression.1,2 Even though most cases present at early stages, in spite of the advances in adjuvant therapy, a significant proportion will develop recurrent metastatic disease.3 In these situations treatment is palliative in nature as most of these patients die as a consequence of progressive disease with a median survival around 2.5 years.4,5
In hormonal receptor negative disease, chemotherapy, anti-angiogenic therapy and anti-HER-2 directed therapy are the main available approaches.6 In these situations, chemotherapy has been unquestionably the basic first line treatment alternative.4-6 However, recent and preliminary but very provocative data suggests impressive results with combinations of targeted agents with no chemotherapy as first line therapy in a phase II trial.7,8 The concomitant blockade of both the anti-angiogenic and the HER-2 growth factor pathways seems to result in a relatively high response rates with a favorable toxicity profile. Other dual pathway blockade strategies are being explored. These will approaches will eventually need validation in randomized trials.
The HER-2 positive population represents approximately 20% of all breast cancer patients. The treatment and the prognosis of this sub-group have been revolutionized with the introduction of the monoclonal antibody Trastuzumab.9,10 While 50% of these patients have hormone receptor negative disease and require anti-HER-2 therapy in combination with cytotoxics, the other 50% do express estrogen or progesterone receptors and recent data suggests that they could be adequately treated with anti-hormonal plus anti-HER2 blockade in the metastatic setting.11
In hormonal receptor positive metastatic disease we frequently face the controversy of initiating therapy with cytotoxics or with endocrine manipulations.4-6 In the very symptomatic patient with a significant volume of disease and visceral compromise the indication of chemotherapy is supported by the perception of a more rapid and higher response rate.12 However, it is important to recognize that the discriminant factor is not the visceral involvement by itself but the clinical and functional consequences of the organ compromise on an individual patient. Endocrine responsive patients with liver or lung metastasis and no or very few clinical symptoms do have both chemotherapy and hormonal therapy as therapeutic alternatives. We should also consider that therapy of metastatic disease intends to palliate symptoms, improve the progression free survival (PFS) and overall survival (OS), improve quality of life and above all, increase or prolong the time to chemotherapy.
There are a number of factors that may help us predict the likelihood of a response to endocrine manipulation. Among those, we can consider a longer disease free interval, soft tissue or bone only disease, an older patient, menopausal status, better performance status, the ER/PR status, and possibly the level of the hormonal receptor expression. By the same token, the absence of these elements may indicate a higher likelihood of benefit with cytotoxic treatment. In this review we address some of the evidence that explores the indication of chemotherapy versus hormonal therapy in hormone receptor positive disease in metastatic breast cancer.
¿ CHEMOTHERAPY RESULTS IN EARLY HORMONAL RECEPTOR POSITIVE BREAST CANCER
The significant improvement in the results obtained with adjuvant therapy in the treatment of early breast cancer over the last few decades should not prevent us to recognize the limitations of this approach. A large body of evidence clearly demonstrates that a significant proportion of patients are cured just with the surgical procedure itself and do not require any further treatment.6 We base our treatment decision in the perceived risk of recurrence and end up treating all or most patients because we cannot identify the ones that require treatment. Furthermore is important to note that another significant portion of patients develop recurrent disease in spite of the adjuvant therapy delivered. These patients are either primarily resistant or eventually develop resistance to our treatment and may actually benefit from therapy by extension of the recurrence-free period.
In early disease, unfortunately, most studies have not been adequately designed to definitively answer the question of the response to chemotherapy in hormone positive early disease. Most of the information available comes from sub-group analysis of large trials that included both hormone positive and negative patients treated with the same approach. In one such subgroup analysis of the yet to be published Intergroup Trial 0100 that randomized patients with positive axillary nodes to receive tamoxifen vs. chemotherapy concomitantly or sequentially with the anti-estrogen, those patients with high estrogen receptor expression derived no benefit from the addition of the chemotherapy.13
In an effort to identify who are the patients that may be spared form adjuvant cytotoxics a retrospective analysis based on the expression of 21 genes was able to select a sub-group comprising 25% of the patients in NSABP B20 that benefited from the introduction of CMF treatment.14 In the other 75% of patients, hormonal therapy alone resulted in the same survival results with no added benefit from the chemotherapy. Analysis of the Intergroup 0100 patients by the same 21 gene platform, has confirmed that a significant proportion of patients with low risk score for recurrence, even though having a worse prognosis do not have added benefit from the addition of FAC chemotherapy.
Other large adjuvant trials and analysis have produced similar results. One retrospective analysis of the CALGB trial that compared AC vs. AC-T suggests that the response to the introduction of paclitaxel to the four cycles of AC backbone was apparent only in the sub-group of patients with negative hormonal receptors.15 The larger subgroup of patients with ER positive and HER2-negative disease did not derive any benefit form the introduction of the taxane.
The neo-adjuvant is another particularly interesting setting where we can explore the differential response to chemotherapy in the hormone positive and hormone negative populations. Unquestionably, a large number of trials show that endocrine receptor negative tumors derive more benefit from chemotherapy as evidenced by a consistent and reproducible higher rate of pathological complete response rate.16-21 MD Anderson investigators recently developed a nomogram to predict the chance of developing a pathological complete response to neo-adjuvant chemotherapy. Estrogen receptor expression was found to be an independent predictor of the pathological response.22 Furthermore; there are suggestions that in ER positive disease, both hormonal and chemotherapy approaches may result in similar rates of pathological complete remissions and breast conserving surgery.23
Finally, a very elegant albeit retrospective review performed by GALGB investigators demonstrates significant differences in the benefits obtained with adjuvant chemotherapy in hormone receptor positive and hormone receptor negative patients.24 Although not including a hormone only treatment group this analysis suggests that most of the benefit derived form adjuvant chemotherapy in sequential trials is observed in the hormonal receptor negative group. This indicates that the effect of adjuvant chemotherapy is different in hormonal receptor positive and negative populations.
It is critical to recognize that most of these results should be considered suggestive as they have been generated retrospectively in subset analysis as previously stated. Even though there is certain consistency in the idea that chemotherapy shows modest activity in hormone positive disease, we should recognize that many of the analyses we have discussed being retrospective do not include the complete sample of cases included in the original trials, there is no report of central hormone receptor testing or review and most importantly, there is no clear prospective definition of what represents a positive test.
To make the issue more complicated some similar analysis of a number of more recently performed trials have not shown a differential effect according to hormonal receptor expression. Among these, the MA.21 study comparing dose dense CEF, EC-T and AC-T, the GEICAM 9906 comparing FE90C vs. FE90C-T, the BCIRG 001 (TAC vs. FAC) and the AGO trail (dose dense ETC vs. EC-T).25-28
It is amazing after so many years and so many trials that this fundamental question remains today with no definitive answer, still controversial and in need of a properly designed randomized clinical trial.
¿ CHEMOTHERAPY RESULTS IN HORMONAL RECEPTOR POSITIVE ADVANCED BREAST CANCER
Just as we have discussed in early disease, it remains surprising that chemotherapy relationship to ER expression has received little attention also in the setting of advanced or metastatic breast cancer. Many randomized trials have included patients irrespective of their hormonal expression status.29-32 Furthermore, the results of the sub-group of patients with receptor positive disease have not been consistently analyzed as a separate group.
If one looks at the criteria used to select metastatic breast cancer patients for inclusion in randomized trials, there has not been a consistent and deliberate attempt to select for a more aggressive phenotype. Even though patients are sometimes selected after being considered hormonally refractory or resistant, or having a short disease-free interval after adjuvant therapy, they have not been selected according to extent of disease or symptoms associated with visceral involvement. We should recognize that this may not be necessarily easy to do. However, it is possible that some natural selection may be in place as patients with slower progression and indolent disease would preferentially be treated or accrued to hormonal manipulation trials or strategies. So it remains difficult to clearly define the proper role of chemotherapy versus hormonal therapy as first line treatment for a significant proportion of patients with advanced breast cancer.
A number of trials and a large meta-analysis have addressed the question with some interesting results. One initial small trial compared a variety of cytotoxic agents and hormonal manipulations in a group of patients with metastatic breast cancer. While the results favored chemotherapy in terms of response rate and survival in the pre-menopausal patients, in the older patients the results were equivalent.33
A larger randomized trial conducted by New Zealand and Australian investigators explored three interesting strategies to treat patients with metastatic breast cancer. The concomitant administration of tamoxifen and doxorubicin plus cyclophosphamide (AC) was compared with the chemotherapy followed by the hormonal treatment upon progression vs. the opposite sequence of tamoxifen followed by AC.34 As expected, the initial response rate to the chemotherapy was higher (45% vs. 22%). Interesting, the response rate for the combination of AC plus Tamoxifen was 51%. However the combined response rate to the sequential approach was similar (42% vs. 46%) for both sequencing arms. More importantly, the survival was almost identical in the three arms with no sub-group showing survival advantage by receiving earlier chemotherapy. The authors conclude that initial hormonal therapy should be an appropriate strategy in this population. In an analysis of adverse prognostic factors for survival, liver involvement, poor PS, prior adjuvant chemotherapy and a short disease free interval were identified as associated with worse results.
In another study addressing again the sequencing issue, investigators treated patients over the age of 65 with tamoxifen or CMF with a crossover design. Response rates were somewhat higher with the anti-estrogen and survivals also favored the tamoxifen group. This trial of hormonal therapy included ER negative patients as well, what makes these results very difficult to interpret.35
Furthermore, most of these earlier studies included hormonal agents and cytotoxic regimens that can be considered outdated by today standards.
The most compelling available evidence addressing this question comes from a meta-analysis performed combining trials conducted form 1963-1995.36 Over 50% of the patients included had visceral metastasis and there was no difference in survival when comparing initial endocrine treatment versus chemotherapy. Little information was presented on safety and tolerability although some of the trials reported more toxicity with the chemotherapy.
With all the caveats and limitations that can be applied to the analysis and the conclusions of these studies, the comparative efficacy of initial chemotherapy or endocrine manipulation in metastatic breast cancer remains uncertain. There is no question that patient selection is critical to help the clinician to solve the question on an individual case. However, it is safe to conclude that in other than the patient with aggressive and rapidly symptomatic visceral metastatic disease that requires chemotherapy, in all other patients initial hormonal therapy remains probably a very good and appropriate option to consider.
¿ TREATMENT OF HORMONAL RECEPTOR-POSITIVE METASTATIC BREAST CANCER WITH HER2 OVER-EXPRESSION
HER-2 over-expression has been associated with more aggressive disease and worse prognosis. Approximately 50% of these patients present with concomitant hormonal receptor expression and are therefore amenable to receive anti-estrogen therapy. On the other hand of all ER positive patients approximately 10% should have HER-2 over-expression. A number of pre-clinical and clinical data suggests that the expression of the growth factor pathway may result in endocrine resistance. In fact one of the proposed mechanisms for the development of tamoxifen resistance is increased signaling through the HER2 family of receptors.37-38
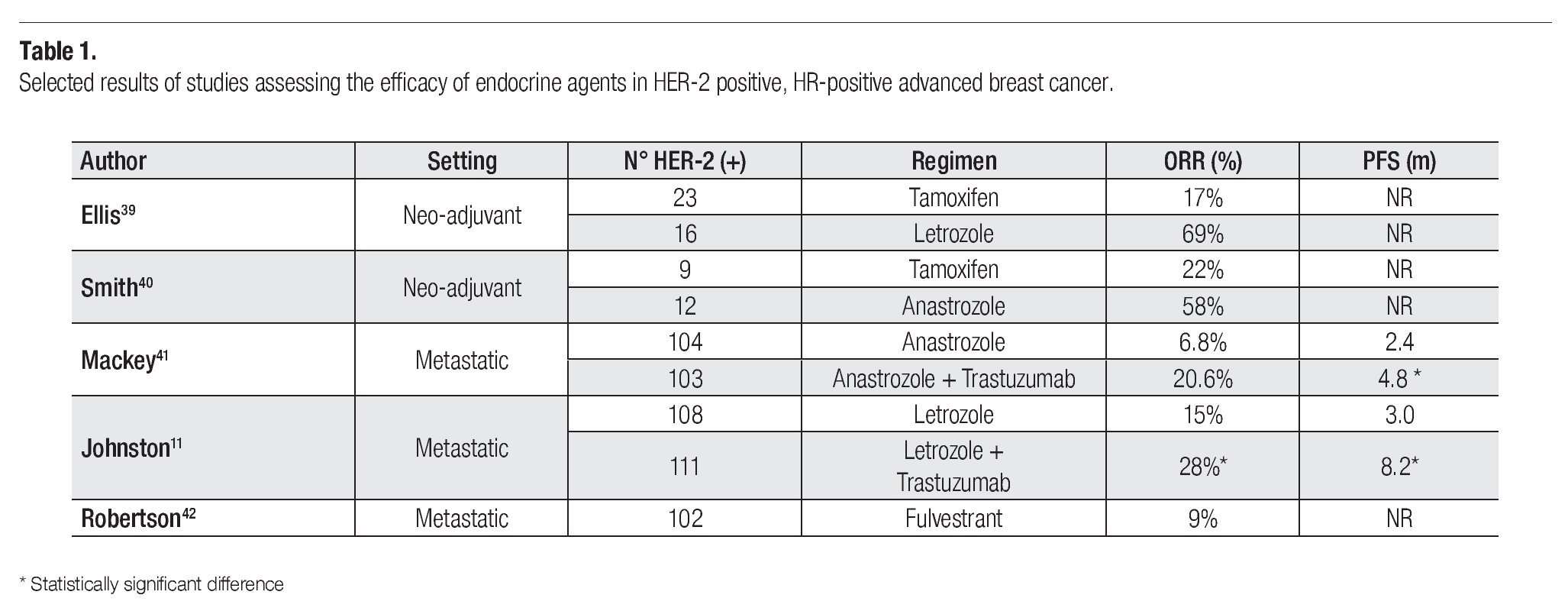
With the introduction of the Aromatase Inhibitors (AI) some studies have explored the comparison of these agents with tamoxifen in this setting (Table 1). As neo-adjuvant treatment, the comparison in the HER-2 population suggests a somewhat higher response to the AIs, although the evidence supporting this conclusion is based in a very small number of patients.39,40
In the metastatic patient with HER-2 over-expression and hormone receptor positive disease there are two important randomized trials to discuss. The first compared anastrozole as a single agent with the combination of anastrozole and trastuzumab in 208 patients.41 This trial showed that the prognosis of patients treated with the hormonal agent alone was very poor, with a progression free survival (PFS) of only 2.4 months. The combination doubled the PFS to 4.8 months but essentially confirmed the poor outcome in this group of patients when treated with trastuzumab and an AI. The response rate was only 6.8% for the anastrozole group suggesting that an inadequate response of these ER expressing HER-2 positive patients to endocrine therapy.
The other study explored the combination of lapatinib, a EGFR/HER-2 tyrosine kinase inhibitor in combination with letrozole versus letrozole as a single agent. The investigators presented data on 1286 randomized patients of which a total of 219 had over-expression of HER-2. The PFS in this last group of patients was significantly prolonged from 3.8 months in the letrozole arm to 8.2 months in the combination (p = 0.019).11 At the same time patients treated with the combination experienced a higher overall response rate (28% vs. 10% respectively; p = 0.021). These results confirm the poor response and prognosis associated with this patient population when treated with single agent aromatase inhibitors. Whether the introduction of chemotherapy would result in better results awaits a more definitive comparison.
Finally, a retrospective pooled analysis from data resulting from 10 different centers explored the estrogen receptor antagonist fulvestrant in a group of 102 patients with HER-2 positive metastatic breast cancer patients. Five of the patients received concomitant trastuzumab and the anti-estrogen. The reported clinical benefit rate (CBR) was 42% (1 CR 8 PR and 34 SD ≥ 6 months). In those patients with CBR the median duration of fulvestrant therapy was 14 months.42
¿ DISCUSSION
Breast cancer is currently recognized as a collection of different diseases with different prognosis and distinct response to available therapies. Gene expression based classifications have initiated and are leading a revolution in the way we approach the disease. It is however very surprising that even though we have been dealing with hormonal receptor expression for so many years we still lack definitive information on the real chemotherapy response in some subgroups of ER and PR positive patients.
Estrogen and progesterone receptor positive breast cancer represent the majority of patients with the disease. In the advanced disease population, the treatment being palliative in nature, the initial choice of therapy should unquestionably consider an endocrine agent. There is no major controversy in the indication of hormonal therapy in the patient with a long disease free interval, slow progressive disease with only bone or soft tissue involvement. However, in the patient with some visceral involvement there are some that believe that chemotherapy should be the selected first alternative. It is important to consider that not all visceral involvement is the same. We should clearly differentiate those patients that present with symptomatic, rapidly progressing lung and or liver involvement were the introduction of cytotoxics may represent the best therapy to manage the disease. Other types of visceral involvement do not necessarily need chemotherapy administration and should be managed with a less toxic hormonal manipulation first. The evidence at hand, even with limitations, suggests that this approach does not compromise long term disease outcome. One the other hand, there is absolutely no evidence indicating that the administration of chemotherapy is essential as the first approach in this group of patients. In the patient with metastatic incurable disease, the delay until the initiation of cytotoxic treatment should be valued as a very worthy endpoint.
One other aspect of this discussion is that considering hormonal receptor expression as a differentiating element in breast cancer we need to accept that the question has not been explored as consistently and appropriately as other aspects of the disease. Furthermore, a critical look at hormonal expression indicates that at the genomic level we can identify at least two different populations, luminal A and B, with distinct clinical behavior and treatment response. Further characterization of these particular sub-groups will certainly help us to identify populations that may be spared of chemotherapy. This seems to be clearly the case in some patients with early disease where we the available evidence indicates no added benefit of chemotherapy when compare to isolated endocrine manipulation.
The evidence at hand clearly suggests that endocrine dependent breast cancer does respond to a lesser degree to cytotoxic treatment as compared to hormonal receptor negative disease. The clinical management of patients with advanced disease should take in consideration this notion. As this review clearly demonstrates, the literature is scarce in definitive information in this regard. Both in early as well as in advanced disease mostly retrospective information indicates that hormone expression decreases the chance for a chemotherapy benefit. However, in view of the limitations of mostly retrospectively generated data, a more definitive conclusion awaits properly conducted clinical trials.
As a clear example on how to go forward in this area, in the HER-2 over-expressing ER/PR positive population, the available phase III data suggests that hormonal therapy by itself does not result in significant benefits and the introduction of at least anti-HER2 therapy and probably chemotherapy should be considered. Similarly designed trials should address the comparative efficacy of chemotherapy and hormones in the population of women with HER2 negative endocrine responsive advanced disease.
Correspondence: Carlos H Barrios.
PUCRS School of Medicine. Pa dre Chagas 66, conj 203, Porto Alegre, RS, Brazil, 90570 080.
Telephone: +55 51 3222 78 52. Fax: +55 51 3346 2217. Cell Phone: +55 51 9840 0425.
E-mail:chbe@via rs.net