The socioeconomic burden of irritable bowel syndrome with constipation (IBS-C) has never been formally assessed in Spain.
Patients and methodsThis 12-month (6-month retrospective and prospective periods) observational, multicentre study assessed the burden of moderate-to-severe IBS-C in Spain. Patients were included if they had been diagnosed with IBS-C (Rome III criteria) within the last 5 years and had moderate-to-severe IBS-C (IBS Symptom Severity Scale score [IBS-SSS] ≥175) at inclusion. The primary objective was to assess the direct cost to the Spanish healthcare system (HS).
ResultsA total of 112 patients were included, 64 (57%) of which had severe IBS-C at inclusion. At baseline, 89 (80%) patients reported abdominal pain and distention. Patient quality of life (QoL), measured by the IBS-C QoL and EQ-5D instruments, was found to be impaired with a mean score of 59 and 57 (0–100, worst-best), respectively. Over the 6-month prospective period the mean IBS-C severity, measured using the IBS-SSS showed some improvement (315–234 [0–500, best–worst]). During the year, 89 (80%) patients used prescription drugs for IBS-C, with laxatives being the most frequently prescribed (n=70; 63%). The direct cost to the HS was €1067, and to the patient was €568 per year. The total direct cost for moderate-to-severe IBS-C was €1635.
DiscussionThe majority of patients reported continuous IBS-C symptoms despite that 80% were taking medication to treat their IBS-C. Overall healthcare resource use and direct costs were asymmetric, with a small group of patients consuming the majority of resources.
El coste socioeconómico del síndrome del intestino irritable con estreñimiento (SII-E) no ha sido evaluado formalmente en España.
Pacientes y métodosEste estudio observacional, multicéntrico a 12 meses (periodos retrospectivo y prospectivo de 6 meses) evaluó el coste del SII-E moderado-grave en España. Se incluyeron pacientes diagnosticados con SII-E (criterios Roma III) en los últimos 5 años y SII-E moderado-grave (puntuación IBS-Symptom Severity Scale [IBS-SSS]≥175) en la inclusión. El objetivo principal fue evaluar el coste directo para el sistema sanitario (SS) español.
ResultadosSe incluyeron un total de 112 pacientes, 64 (57%) de los cuales presentaban SII-E grave en la inclusión. En el momento basal, 89 (80%) pacientes presentaron dolor y distensión abdominal. La calidad de vida del paciente, medida mediante los instrumentos IBS-C QoL y EQ-5D, estaba deteriorada, con una puntuación de 59 y 57 (0-100, peor-mejor), respectivamente. En el periodo prospectivo la gravedad media del SII-E, medida mediante IBS-SSS, mostró alguna mejoría (315 a 234 [0-500, mejor-peor]). Durante el periodo a studio, 89 (80%) pacientes usaron fármacos prescritos para el SII-E, principalmente laxantes (n=70; 63%). El coste directo anual fue de 1.067€ y 568€ para el SS y el paciente, respectivamente. El coste total directo del SII-E moderado-grave fue de 1.635€.
DiscusiónLa mayoría de pacientes presentaron síntomas continuos del SII-E pese a que el 80% tomaban medicación específica. El uso de recursos sanitarios y los costes directos globales fueron asimétricos, con un pequeño grupo de pacientes consumiendo la mayoría de los recursos.
Irritable bowel syndrome (IBS) is a chronic functional bowel disorder with an estimated prevalence of 12% in Spain and between 5% and 20% worldwide.1–3 It is characterised by recurrent symptoms of abdominal pain accompanied by altered bowel function.4 Subdivision of IBS by the Rome III criteria lists four subtypes: IBS with diarrhoea (IBS-D), IBS with constipation (IBS-C), mixed IBS (IBS-M), and unsubtyped IBS (IBS-U).5 Disease activity can vary over time, with periods of high disease activity followed by periods of remission.6 Similarly, symptoms can change over time and almost half (40%) of IBS patients can transition from one subtype to another.7
IBS has been shown to negatively impact quality of life (QoL), for example, affecting sleep, diet, personal/work relationships, and sexual function.5,8 Moderate to severe IBS account for approximately 60% of all IBS cases and previous studies have shown a substantial economic burden to society through direct medical costs and indirect societal costs.9–11
According to the Rome III criteria, the diagnostic criteria for IBS is recurrent abdominal pain or discomfort present for at least three days/month in the last three months, accompanied by two or more of the following: improvement with defecation, onset associated with a change in stool frequency, or a change in stool form.12 For IBS-C, these characteristics are accompanied by ≥25% of bowel movements being hard or lumpy stools and <25% of bowel movements being loose or watery stools. The IBS-C subtype is characterised by constipation, and commonly underdiagnosed due to its similarity to chronic constipation as it shares similar defecation patterns accompanied by occasional abdominal discomfort.13 Anyway, it is thought that IBS-C affects about 30% of the IBS population. Rome IV criteria, published in 2016, included some changes when compared to Rome III criteria.14 Nevertheless, no major differences in severity evaluation are anticipated.
Some studies suggest that the current burden of illness for IBS is quite significant.8,15–17 Traditional therapies including laxatives, prokinetics, antispasmodics, and bulking agents (such as dietary fibres) are useful for treating constipation in some patients, however, their use is limited due to low overall efficacy and tolerability that is compounded by the fact that as individual treatments they do not treat all the key IBS-C symptoms.18,19 Thus, there remains a clear need for more effective therapeutic agents for the treatment of IBS-C. To date, linaclotide – a guanylate cyclase-C agonist – is the only licensed pharmacological treatment in Europe for the treatment of IBS-C.20
Therefore, the aim of this study was to describe the socioeconomic burden of moderate to severe IBS-C. Here we report the QoL of patients with IBS-C, the evolution of IBS-C severity over time, and the direct economic costs of IBS-C in Spain.
Patients and methodsThis report is the Spanish subset analyses of an observational, 12-month, multicentre study with 6-month retrospective and 6-month prospective components, conducted in 6 European countries. The first patient was included in April 2012 and the last patient last visit was in January 2014. Patients were included in the study if they were ≥18 years of age, diagnosed with IBS-C in the last 5 years using the Rome III criteria (Rome IV criteria were not published at that time), and had moderate to severe IBS-C at inclusion, defined as an IBS Symptom Severity Scale (IBS-SSS) score ≥175. The IBS-SSS score was obtained by the sum of the five equally-weighted questions related to pain, distention, bowel dysfunction, and general well-being. Each question is scored out of 100; moderate severity is defined as an overall score of 175–300; and severe severity as >300.21 Patients were excluded if they had participated in a clinical trial involving an experimental IBS-C treatment in the 6 months prior to starting the observational period, or they had an acute or chronic condition that, in the investigator's opinion, would impact the patient's ability to complete the study. This study was conducted in accordance with the Declaration of Helsinki as well as in compliance with ICH GCP guidelines. All ethics committees approved the trial protocol and its amendments.
Patients were screened using medical records in primary and specialist care. Baseline and retrospective data of patients enrolled in the study were obtained from patient interviews and patient medical records, respectively. Demographic, clinical, and QoL data were collected at baseline. Assessment of QoL was performed using the IBS-QoL22 and EQ-5D23 instruments. The IBS-QoL included an overall score and eight subscale scores, with 0 indicating the worst QoL and 100 indicating the highest possible QoL. The EQ-5D was a generic measure of health status and consisted of two parts. The first part consisted of five categorical dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression; these were scored on a 3-point Likert scale. The second part consisted of a visual analogue scale (VAS) where 0 indicated the worst health state and 100 indicated the best health state.
Symptom severity was collected using the IBS-SSS at baseline, and at 3 and 6 months. Healthcare resource utilisation (HRU) data related to IBS-C (medical consultations, hospitalisations, diagnostic tests, therapies, management of adverse reactions) were collected using a questionnaire (specifying whether the costs were public or private). A maximum of 6 months’ direct HRU costs were collected at baseline retrospectively. Prospective HRU data were collected during routine follow-up at 3 months (±0.5 months) and 6 months (±1 month)/early termination. All HRU data were calculated in patients that used the resource.
Direct costs were calculated for the Spanish healthcare system (HS) and the patient's perspective. Consultations, physician visits, and diagnostic tests were estimated from the Listados de Boletines Oficiales de Tarifas de Comunidades Autónomas (2005–2011). Hospitalisation costs were estimated from Grupos Relacionados con el Diagnóstico (GRD/DRG) correspondientes and estadísticas. Ministerio de Sanidad, Servicios Sociales e Igualdad (Tarifa AP25, 2008), and Listados de Boletines Oficiales de tarifas de Comunidades Autónomas (2005–2011). Drug costs were estimated from the Ministerio de Sanidad, Servicios Sociales e Igualdad (Nomenclator Digitalis-Integra) and the Consejo General de Colegios Oficiales de Farmacéuticos (Bot Plus, Portalfarma). For the patient's perspective, only the sum of non-prescription medication, complementary therapies, the percentage of HS medications, consultations, hospitalisations, and diagnostic procedures paid for by the patient were taken into account; costs of private consultations and diagnostic procedures were not included.
The analyses performed were only exploratory. Demographics, baseline characteristics, HRU characteristics and productivity losses were summarised using descriptive statistics based on non-missing observations. Costs were calculated as a mean with 95% confidence interval (calculated using 1000 bootstrap samples).
The retrospective, prospective, and combined data periods were analysed separately. The separate analyses were then compared to determine whether there was any statistical difference between them. The data presented here is derived from the combined analysis.
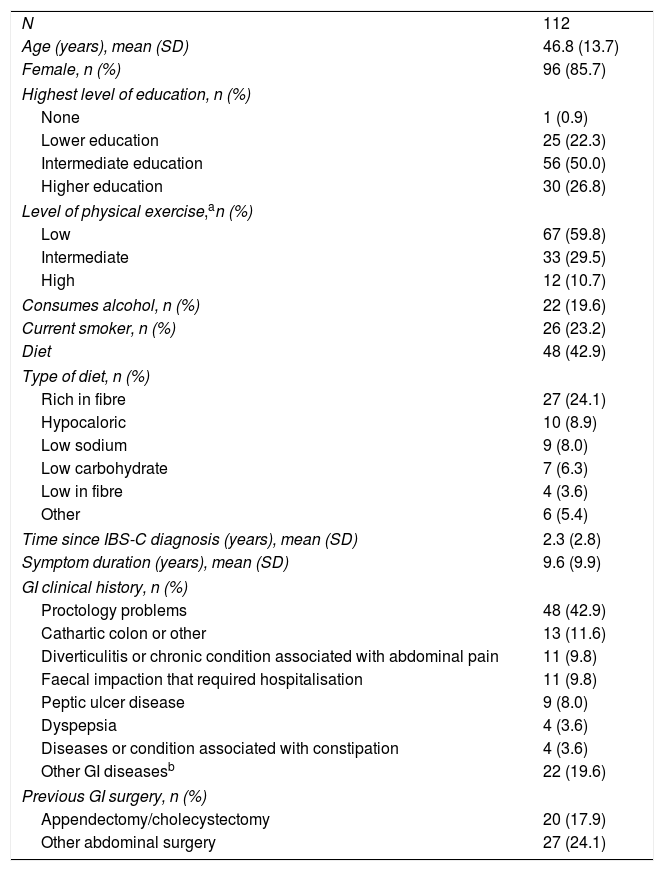
ResultsOne hundred and twelve patients were enrolled from 4 primary care centres and 12 specialist centres in Spain (Table 1). Over the six-month prospective follow-up period, there were 7 (6.3%) discontinuations: 6 (5.4%) patients were lost to follow-up and 1 (0.9%) withdrew due to serious illness.
Patient demographics.
| N | 112 |
| Age (years), mean (SD) | 46.8 (13.7) |
| Female, n (%) | 96 (85.7) |
| Highest level of education, n (%) | |
| None | 1 (0.9) |
| Lower education | 25 (22.3) |
| Intermediate education | 56 (50.0) |
| Higher education | 30 (26.8) |
| Level of physical exercise,an (%) | |
| Low | 67 (59.8) |
| Intermediate | 33 (29.5) |
| High | 12 (10.7) |
| Consumes alcohol, n (%) | 22 (19.6) |
| Current smoker, n (%) | 26 (23.2) |
| Diet | 48 (42.9) |
| Type of diet, n (%) | |
| Rich in fibre | 27 (24.1) |
| Hypocaloric | 10 (8.9) |
| Low sodium | 9 (8.0) |
| Low carbohydrate | 7 (6.3) |
| Low in fibre | 4 (3.6) |
| Other | 6 (5.4) |
| Time since IBS-C diagnosis (years), mean (SD) | 2.3 (2.8) |
| Symptom duration (years), mean (SD) | 9.6 (9.9) |
| GI clinical history, n (%) | |
| Proctology problems | 48 (42.9) |
| Cathartic colon or other | 13 (11.6) |
| Diverticulitis or chronic condition associated with abdominal pain | 11 (9.8) |
| Faecal impaction that required hospitalisation | 11 (9.8) |
| Peptic ulcer disease | 9 (8.0) |
| Dyspepsia | 4 (3.6) |
| Diseases or condition associated with constipation | 4 (3.6) |
| Other GI diseasesb | 22 (19.6) |
| Previous GI surgery, n (%) | |
| Appendectomy/cholecystectomy | 20 (17.9) |
| Other abdominal surgery | 27 (24.1) |
Out of the 112 patients enrolled in the study, 25 (22.3%) were recruited from primary care centres (all public), whereas 87 (77.7%) were recruited from specialist centres (59 [52.7%] from public centres). Baseline patient demographics are shown in Table 1. The average time since IBS-C diagnosis was 2.3 years, with an IBS-C symptom duration of 9.6 years. Twenty (17.9%) patients had undergone a prior appendectomy/cholecystectomy, and 27 (24.1%) patients had some other form of prior abdominal surgery.
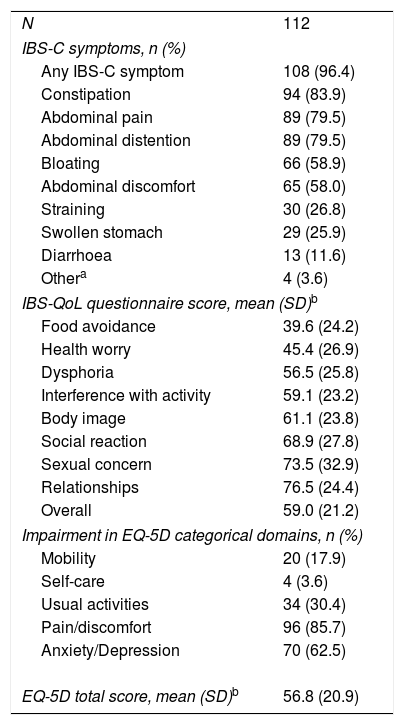
IBS-C symptoms and quality of life at baselineAt baseline, 108 (96.4%) patients reported IBS symptoms (Table 2). Constipation (n=94; 83.9%), abdominal pain (n=89; 79.5%), and abdominal distention (n=89; 79.5%) were the most frequently reported symptoms. Sixty-six (58.9%) and 65 (58.0%) patients reported suffering from bloating and abdominal discomfort, respectively. Using the IBS-QoL instrument, the most affected domains were food avoidance and health worry. The overall impact of IBS-C on QoL was similar for the IBS-QoL and EQ-5D questionnaires, 59.0 and 56.8 respectively. In addition, 96 (85.7%) patients reporting moderate to severe pain/discomfort, with 70 (62.5%) reporting moderate to severe anxiety/depression through the EQ-5D categorical dimensions.
IBS-C symptoms and quality of life at baseline.
| N | 112 |
| IBS-C symptoms, n (%) | |
| Any IBS-C symptom | 108 (96.4) |
| Constipation | 94 (83.9) |
| Abdominal pain | 89 (79.5) |
| Abdominal distention | 89 (79.5) |
| Bloating | 66 (58.9) |
| Abdominal discomfort | 65 (58.0) |
| Straining | 30 (26.8) |
| Swollen stomach | 29 (25.9) |
| Diarrhoea | 13 (11.6) |
| Othera | 4 (3.6) |
| IBS-QoL questionnaire score, mean (SD)b | |
| Food avoidance | 39.6 (24.2) |
| Health worry | 45.4 (26.9) |
| Dysphoria | 56.5 (25.8) |
| Interference with activity | 59.1 (23.2) |
| Body image | 61.1 (23.8) |
| Social reaction | 68.9 (27.8) |
| Sexual concern | 73.5 (32.9) |
| Relationships | 76.5 (24.4) |
| Overall | 59.0 (21.2) |
| Impairment in EQ-5D categorical domains, n (%) | |
| Mobility | 20 (17.9) |
| Self-care | 4 (3.6) |
| Usual activities | 34 (30.4) |
| Pain/discomfort | 96 (85.7) |
| Anxiety/Depression | 70 (62.5) |
| EQ-5D total score, mean (SD)b | 56.8 (20.9) |
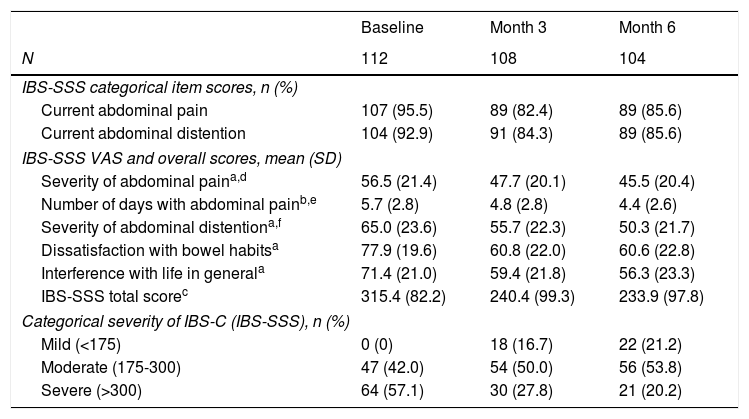
The IBS-C severity was assessed three times over the prospective period at three-month intervals. The number of patients reporting current abdominal pain and current abdominal distention decreased from 95.5% and 92.9% (respectively) at baseline to 85.6% at 6 months for both categories (Table 3). According to the IBS-SSS VAS, the severity of abdominal pain showed the lower decrease changing from mean±SD 56.5±21.4 at baseline to 45.5±20.4 at 6 months. By contrast, the number of days with abdominal pain and the severity of abdominal pain exhibited the largest reduction, from 5.7±2.8 days and 65.0±23.6 to 4.4±2.6 days and 50.3±21.7, respectively. Overall, the total score decreased by 26%, from 515.4±82.2 to 233.9±97.8, decreasing accordingly the mean severity grade from “severe” at baseline to “moderate” at 6 months. Categorically, this observation is demonstrated by the overall reduction in patients with severe IBS-C – from 64 (57.1%) at baseline to 21 (20.2%) at 6 months. By contrast, both the number of patients with moderate and mild severity at baseline and 6 months increased from 47 (42.0%) to 56 (53.8%) and from 0 to 22 (21.2%), respectively.
Evolution of IBS-C severity.
| Baseline | Month 3 | Month 6 | |
|---|---|---|---|
| N | 112 | 108 | 104 |
| IBS-SSS categorical item scores, n (%) | |||
| Current abdominal pain | 107 (95.5) | 89 (82.4) | 89 (85.6) |
| Current abdominal distention | 104 (92.9) | 91 (84.3) | 89 (85.6) |
| IBS-SSS VAS and overall scores, mean (SD) | |||
| Severity of abdominal paina,d | 56.5 (21.4) | 47.7 (20.1) | 45.5 (20.4) |
| Number of days with abdominal painb,e | 5.7 (2.8) | 4.8 (2.8) | 4.4 (2.6) |
| Severity of abdominal distentiona,f | 65.0 (23.6) | 55.7 (22.3) | 50.3 (21.7) |
| Dissatisfaction with bowel habitsa | 77.9 (19.6) | 60.8 (22.0) | 60.6 (22.8) |
| Interference with life in generala | 71.4 (21.0) | 59.4 (21.8) | 56.3 (23.3) |
| IBS-SSS total scorec | 315.4 (82.2) | 240.4 (99.3) | 233.9 (97.8) |
| Categorical severity of IBS-C (IBS-SSS), n (%) | |||
| Mild (<175) | 0 (0) | 18 (16.7) | 22 (21.2) |
| Moderate (175-300) | 47 (42.0) | 54 (50.0) | 56 (53.8) |
| Severe (>300) | 64 (57.1) | 30 (27.8) | 21 (20.2) |
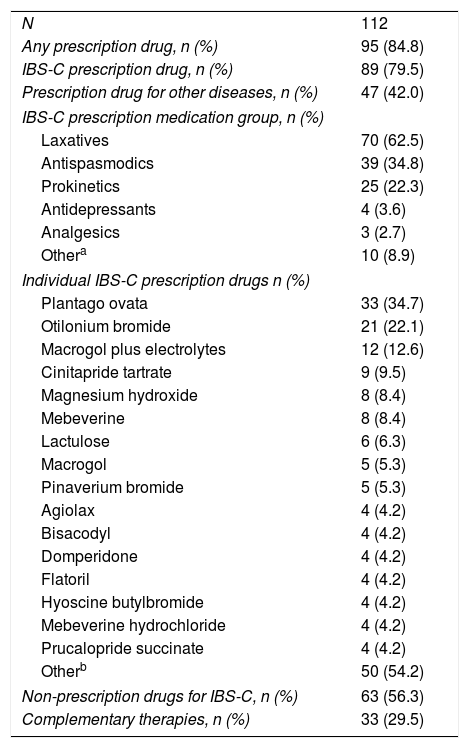
Overall, 95 (84.8%) patients took some form of prescription medication over the 12-month study period (Table 4). Eighty-nine patients (79.5%) took prescription medication to treat their IBS-C whereas 47 (42.0%) patients took prescription medication to treat another disease. The most frequently prescribed medication class for IBS-C was laxatives in 70 (62.5%) patients. Antispasmodics and prokinetics were the second and third most prescribed medication classes at 39 (34.8%) and 25 (22.3%) patients, respectively. In terms of individual medications, the most frequently prescribed medication was plantago ovata, followed by otilonium bromide, and macrogol plus electrolytes. Sixty-three (56.3%) patients took non-prescription drugs, whereas 33 (29.5%) sought complementary therapies.
Medication use over 12 months.
| N | 112 |
| Any prescription drug, n (%) | 95 (84.8) |
| IBS-C prescription drug, n (%) | 89 (79.5) |
| Prescription drug for other diseases, n (%) | 47 (42.0) |
| IBS-C prescription medication group, n (%) | |
| Laxatives | 70 (62.5) |
| Antispasmodics | 39 (34.8) |
| Prokinetics | 25 (22.3) |
| Antidepressants | 4 (3.6) |
| Analgesics | 3 (2.7) |
| Othera | 10 (8.9) |
| Individual IBS-C prescription drugs n (%) | |
| Plantago ovata | 33 (34.7) |
| Otilonium bromide | 21 (22.1) |
| Macrogol plus electrolytes | 12 (12.6) |
| Cinitapride tartrate | 9 (9.5) |
| Magnesium hydroxide | 8 (8.4) |
| Mebeverine | 8 (8.4) |
| Lactulose | 6 (6.3) |
| Macrogol | 5 (5.3) |
| Pinaverium bromide | 5 (5.3) |
| Agiolax | 4 (4.2) |
| Bisacodyl | 4 (4.2) |
| Domperidone | 4 (4.2) |
| Flatoril | 4 (4.2) |
| Hyoscine butylbromide | 4 (4.2) |
| Mebeverine hydrochloride | 4 (4.2) |
| Prucalopride succinate | 4 (4.2) |
| Otherb | 50 (54.2) |
| Non-prescription drugs for IBS-C, n (%) | 63 (56.3) |
| Complementary therapies, n (%) | 33 (29.5) |
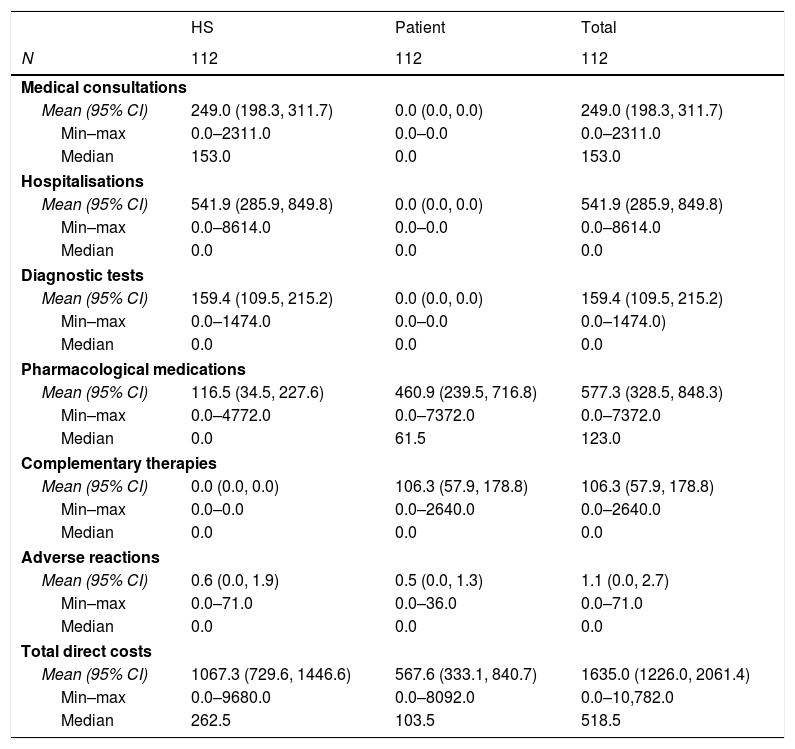
The direct cost of IBS-C was estimated for the HS and for the patient (Table 5). For the HS the largest cost drivers were hospitalisations and medical consultations, which accounted for 50.8% and 23.3% of the total costs, respectively. By contrast, pharmacological medications and complementary therapies were the largest drivers for costs to the patients, at 81.2% and 18.7% of the total (€567.6), respectively. Overall, pharmacological medications and hospitalisations accounted for 35.3% and 33.1% of the total direct costs. The total cost of moderate to severe IBS-C included €1635 of direct costs, with patients paying 34.7% of these costs. A comparison of retrospective and prospective data revealed no significant differences between the retrospective and prospective periods.
Direct costs of IBS-C.
| HS | Patient | Total | |
|---|---|---|---|
| N | 112 | 112 | 112 |
| Medical consultations | |||
| Mean (95% CI) | 249.0 (198.3, 311.7) | 0.0 (0.0, 0.0) | 249.0 (198.3, 311.7) |
| Min–max | 0.0–2311.0 | 0.0–0.0 | 0.0–2311.0 |
| Median | 153.0 | 0.0 | 153.0 |
| Hospitalisations | |||
| Mean (95% CI) | 541.9 (285.9, 849.8) | 0.0 (0.0, 0.0) | 541.9 (285.9, 849.8) |
| Min–max | 0.0–8614.0 | 0.0–0.0 | 0.0–8614.0 |
| Median | 0.0 | 0.0 | 0.0 |
| Diagnostic tests | |||
| Mean (95% CI) | 159.4 (109.5, 215.2) | 0.0 (0.0, 0.0) | 159.4 (109.5, 215.2) |
| Min–max | 0.0–1474.0 | 0.0–0.0 | 0.0–1474.0) |
| Median | 0.0 | 0.0 | 0.0 |
| Pharmacological medications | |||
| Mean (95% CI) | 116.5 (34.5, 227.6) | 460.9 (239.5, 716.8) | 577.3 (328.5, 848.3) |
| Min–max | 0.0–4772.0 | 0.0–7372.0 | 0.0–7372.0 |
| Median | 0.0 | 61.5 | 123.0 |
| Complementary therapies | |||
| Mean (95% CI) | 0.0 (0.0, 0.0) | 106.3 (57.9, 178.8) | 106.3 (57.9, 178.8) |
| Min–max | 0.0–0.0 | 0.0–2640.0 | 0.0–2640.0 |
| Median | 0.0 | 0.0 | 0.0 |
| Adverse reactions | |||
| Mean (95% CI) | 0.6 (0.0, 1.9) | 0.5 (0.0, 1.3) | 1.1 (0.0, 2.7) |
| Min–max | 0.0–71.0 | 0.0–36.0 | 0.0–71.0 |
| Median | 0.0 | 0.0 | 0.0 |
| Total direct costs | |||
| Mean (95% CI) | 1067.3 (729.6, 1446.6) | 567.6 (333.1, 840.7) | 1635.0 (1226.0, 2061.4) |
| Min–max | 0.0–9680.0 | 0.0–8092.0 | 0.0–10,782.0 |
| Median | 262.5 | 103.5 | 518.5 |
HS: healthcare system; CI: confidence interval.
NOTE: Costs expressed in €.
This is the first study directly evaluating the socioeconomic impact of moderate to severe IBS-C in Spain. As defined by the Rome III criteria, a positive diagnosis requires IBS-C symptoms to be present for three days per month every three months. During enrolment, we observed that almost all patients were currently reporting one or more IBS-C symptom, indicating that severe patients have a high symptom frequency. Regarding QoL, moderate to severe IBS-C patients reported a greater overall QoL impairment compared to previous studies including IBS patients of all subtypes and severities.8,17,24 Studies that predominantly included moderate or severe IBS patients showed a similar overall QoL score to the herein reported.25–27 The assessment of the individual IBS-QoL instrument questions mirrored previous observations indicating that the largest impairments in QoL are manifested in terms of dietary restrictions and concern about health.8,17,24–26 Similar to the IBS-QoL, the mean score in the EQ-5D VAS was at the lower end of that previously reported for IBS patients (55.3–61.4).8,17,28 For the categorical items, similar to the aforementioned studies, few patients reported problems with self-care. The most frequently reported problems were pain/discomfort and anxiety/depression.28 These figures, combined with the high degree of health worry derived from the IBS-QoL instrument, support the confirmation of IBS-C as having an important psychological impact.
At baseline, the mean IBS-SSS score indicated a great severity, with an interference with life in general and a dissatisfaction with bowel habits being the most severely affected categories. This was also observed in the categorical scores from an all-subtype IBS study with a similar overall IBS-SSS score.29 Over follow-up, improvements in IBS-C severity were similar to that observed in previous studies.30,31 The most likely reason for improvement without a specific change in intervention reflects a regression towards the mean and the waxing and waning nature of the disorder.
Prescription drug use was also high for moderate to severe IBS-C patients. Surprisingly, IBS-C related medication use was only slightly higher than that previously reported for moderate to severe all-subtype IBS patients, and yet marginally lower than that reported for IBS patients of all subtypes and severities.25 Non-prescription drugs were also taken by over half of the patients, and an additional third sought complementary therapies, thus suggesting dissatisfaction with current prescription medications.
The largest cost driver for the HS was hospitalisations/emergency room (ER) visits, which was two-fold above the second cost driver – medical consultations – and over three-fold above other costs. Hospitalisations account for 50% of HS costs, approximately two-fold higher than those previously reported for mixed-severity/all-subtype French IBS patients.15 Thus, this suggests that moderate to severe IBS-C patients require more inpatient care compared to all-severity patients. Further comparisons from other countries will be necessary to verify this observation.
Costs to the patient were lower, at approximately half the HS’ direct costs. The largest direct costs paid by the patient were medication costs (81.2%), followed by complementary therapies (18.7%). Approximately two-thirds of the total direct costs for IBS-C in Spain were attributable to the HS, with the remaining third being attributable to the patient. The largest drivers of total direct costs for IBS-C in moderate to severe patients were medication costs (35.3%), hospitalisations/ER visits (33.1%), and consultation costs (15.2%). Overall, the costs for hospitalisations/ER visits accounted for a larger proportion of costs than previously reported for mixed-severity IBS/IBS-C patients.3,15,16 It is worth noting that the proportion of overall costs attributable to each cost component varied widely between studies and are most likely explained by both the differences in therapeutic management and in reimbursement policies.
In comparison to previous European studies for IBS patients across all severities, costs related to moderate-severe patients shown here were substantially higher.3,11,15–17
The main limitations of this study were the possibility of healthcare resource underestimation due to approximately three quarters of patients being recruited from specialist care centres. Cost estimations may also be underestimated due to the exclusion of private consultation and diagnostic procedure costs. Furthermore, the partially retrospective nature of the study may be associated with some recall bias.
ConclusionWe observed a high socioeconomic impact of moderate to severe IBS-C in Spain. Despite 80% of patients taking medication to treat their IBS-C, the majority of patients reported continued IBS-C symptoms. This negatively impacted QoL with a high percentage of patients reporting pain/discomfort and anxiety/depression. The variety of medications prescribed to patients, in combination with a high degree of non-prescription medication and complementary therapy use, suggest that patients are not controlled with currently available therapeutic medications. From an economic perspective, the overall direct cost was highly asymmetrical with few patients consuming the major part of healthcare resources. Together, poor QoL and high resource use demonstrates the high socioeconomic cost of this chronic functional bowel disorder.
Declaration of author contributionsF. Mearin was the coordinating investigator in Spain and J. Tack was the lead coordinator of the study. A.M. Caballero, J. Serra, C. Brotons, A. Tantiñà, E. Fort, F.J. Martínez-Cerezo, A. Perelló, G. Sánchez-Antolín, E. Rey, R. Angós Musgo, R. Berdier, B. Gómez-Rodríguez, P. Clavé, M. García-Alonso, and P. Torán-Monserrat contributed to clinical data collection. All authors contributed to the creation of the manuscript and all authors revised and approved the final version of the manuscript.
Declaration of fundingThis study and all data analyses were funded in full by Almirall, S.A. The preparation of this paper was funded by Almirall, S.A and Allergan International.
Conflicts of interestF. Mearin receives speaker fees from Almirall and J. Tack receives grants and/or research support from Abbott, Novartis and Shire; honoraria and/or consultancy fees from Almirall, AstraZeneca, Danone, GI Dyamics, GlaxoSmithKline, Ironwood, Janssen, Menarini, Novartis, Rhythm, Shire, Takeda, Theravance, Tsumura, Will Pharma and Zeria; and speaker fees from Abbott, Almirall, AstraZeneca, Janssen, Menarini, Novartis, Shire, Takeda and Zeria. F.J. Martínez-Cerezo received speaker fees from Abbie, Casen-Fleet, Gilead and Janssen; and grants and/or research support from Abbie and MSD. The remaining authors declare no conflicts of interest.
The authors would like to thank the IBIS-C study patients for their participation in this trial. Writing support was provided by Jonathan Mackinnon, PhD from TFS Develop.