Rapid Urease Test (RUT) is a simple, cheap and relatively fast method for diagnosing Helicobacter pylori infection. It is therefore the preferred method used for patients undergoing gastroscopy. Most kits require 24h to give results. The new Ultra-Rapid Urease Test (URUT) kit by Biohit® requires less than 1h.
ObjectiveTo determine URUT's diagnostic accuracy.
MethodProspective, blind, multi-centre study involving dyspeptic patients. One corpus biopsy and three antral biopsies were obtained during gastroscopy for standard histological analysis, RUT and URUT. The URUT result was checked after 1min, 5min, 30min and 60min and the RUT was checked over the course of 24h. Histology was used as the gold standard test.
Results144 patients were included, 68% female, with a mean age of 49 years old; 50% were H. pylori positive. RUT and URUT diagnoses were correct in 85.9% and 90% of the cases, respectively. The mean waiting time for a positive RUT result was 6h. The sensitivity, specificity, and positive and negative predictive values for RUT were, respectively, 82%, 90%, 89% and 84%. The URUT's results were similar (85%, 94%, 94% and 87%). These figures improved when patients taking PPIs were excluded (RUT: 86%, 91%, 93% and 83%; URUT: 91%, 94%, 96% and 89%). No statistically significant differences were found when comparing RUT and URUT distributions of correct diagnoses (McNemar's Test, p=0.3) but there was a tendency towards better results with the URUT.
ConclusionThe URUT is equivalent to (or slightly better than) the traditional RUT in diagnosing H. pylori infection, and provides results in less than an hour.
El test de la ureasa (TRU) es un método simple, barato y relativamente rápido para el diagnóstico de la infección por Helicobacter pylori (H. pylori). Por tanto, es el método de elección en pacientes sometidos a gastroscopia. La mayoría de los kits requieren 24 h para obtener un resultado. En nuevo test ultrarrápido de la ureasa (TURU) de Biohit requiere menos de una hora.
ObjetivoDeterminar la exactitud diagnóstica del TURU.
MétodoEstudio multicéntrico, prospectivo y ciego, en el que se incluyó a pacientes dispépticos. Se obtuvieron 3 biopsias de antro y una de corpus durante la gastroscopia para análisis histológico estándar, TRU y TURU. El resultado del TURU se comprobó a los 1, 5, 30 y 60 min, mientras que el TRU se evaluó a lo largo de 24 h. La histología se utilizó como patrón oro.
ResultadosSe incluyó a 144 pacientes, 68% mujeres, edad media 49 años, el 50% fueron positivos para H. pylori. TRU y TURU diagnosticaron correctamente el 85,9% y 90,0% de los casos, respectivamente. La duración media de espera para un resultado positivo del TRU fue 6 h. La sensibilidad, la especificidad y los valores predictivos negativo y positivo para el TRU fueron, respectivamente, del 82, el 90, el 89 y el 84%. Los resultados del TURU fueron equivalentes (el 85, el 94, el 94 y el 87%). Estos resultados mejoraron al excluir los pacientes que tomaban IBP (TRU: 86, 91, 93 y 83%; TURU: 91, 94, 96 y 89%). La comparación de distribución de diagnósticos correctos entre TRU y TURU no encontró diferencias estadísticamente significativas (test de McNemar p=0,3) pero existe una tendencia a mejores resultados con el TURU.
ConclusiónEl TURU es equivalente (o algo superior) al TRU tradicional en el diagnóstico de la infección por H. pylori y obtiene los resultados en menos de una hora.
The management of Helicobacter pylori (H. pylori) infection requires accurate diagnostic methods. Several diagnostic methods, both direct (histology, culture and urease tests) and indirect (faecal antigen, serology and urea breath test), have been developed.1–4 Direct or “invasive” methods require the biopsy sampling while indirect or “non-invasive” methods detect secondary characteristics of the bacteria.5
Patients undergoing gastroscopy for dyspeptic symptoms are generally tested for H. pylori infection. Rapid Urease Test (RUT) is usually the method of choice as it is simple, cheap, gives an accurate result in 24h and the result can be observed in the endoscopic units without the involvement of other services.3–6 Urease kits have a media containing urea and a pH-dependant colour indicator, in which the biopsy is placed. H. pylori urease activity alters the pH causing a change of colour of the media.7
Common RUTs require up to 24h to obtain an accurate result, what limits its benefits and utility in clinical practice as the treatment prescription cannot be given in the moment, forcing the patient to come back for the diagnosis to the hospital's outpatient clinic.1,8 A quicker urease kit would increase efficiency by reducing costs and incommodities to patients.
Recently, new kits have been developed trying to improve the utility and speed of the urease method.5–7,9 Biohit® commercializes a new urease method that, according to its technical information, is able to give accurate results in 30min. Previous studies have evaluated the accuracy of different commercial kits obtaining encouraging results.10–17 It seems that Biohit®’s Ultra-Rapid Urease Test (URUT) is a promising diagnostic method but wider and more diverse evidence is still needed to recommend the systematic use URUT in clinical practice. Therefore, the aim of the present study was to evaluate and compare URUTs diagnostic accuracy for the diagnosis of H. pylori in the Spanish population.
MethodsPatientsIn this prospective, blind, multicenter study, a total of 144 patients (68% female, mean age 49 years) who attended digestive services for upper gastrointestinal endoscopy were consecutively enrolled. Inclusion criteria were: patients over 18 years of age suffering from dyspepsia. Exclusion criteria were: presence of hepatic, renal, lung, endocrine, metabolic, haematological or malignant diseases; previous H. pylori eradication treatment; history of alcohol or drug abuse; and pregnancy or nursing.
Proton pump inhibitor (PPI) treatment was not considered an exclusion criterion as, in clinical practice, most patients undergoing upper gastrointestinal endoscopy are taking these drugs prior to the procedure. However, sub-analyses depending on PPI intake were performed for all calculations.
The investigator in charge of each diagnostic test was blinded to the results of the other tests.
BiopsiesThree antrum and one corpus biopsies were obtained. One antrum and the corpus biopsies were used for the standard histological analysis. Biopsies were fixed in 10% formalin and separately embedded in paraffin blocks. The sections, serially cut and stained with haematoxylin & eosin, were examined with light microscopy for the histological assessment of H. pylori infection by a pathologist. One antrum biopsy was used for the diagnosis of H. pylori infection with the traditional RUT. The other biopsy was used for diagnosis with URUT.
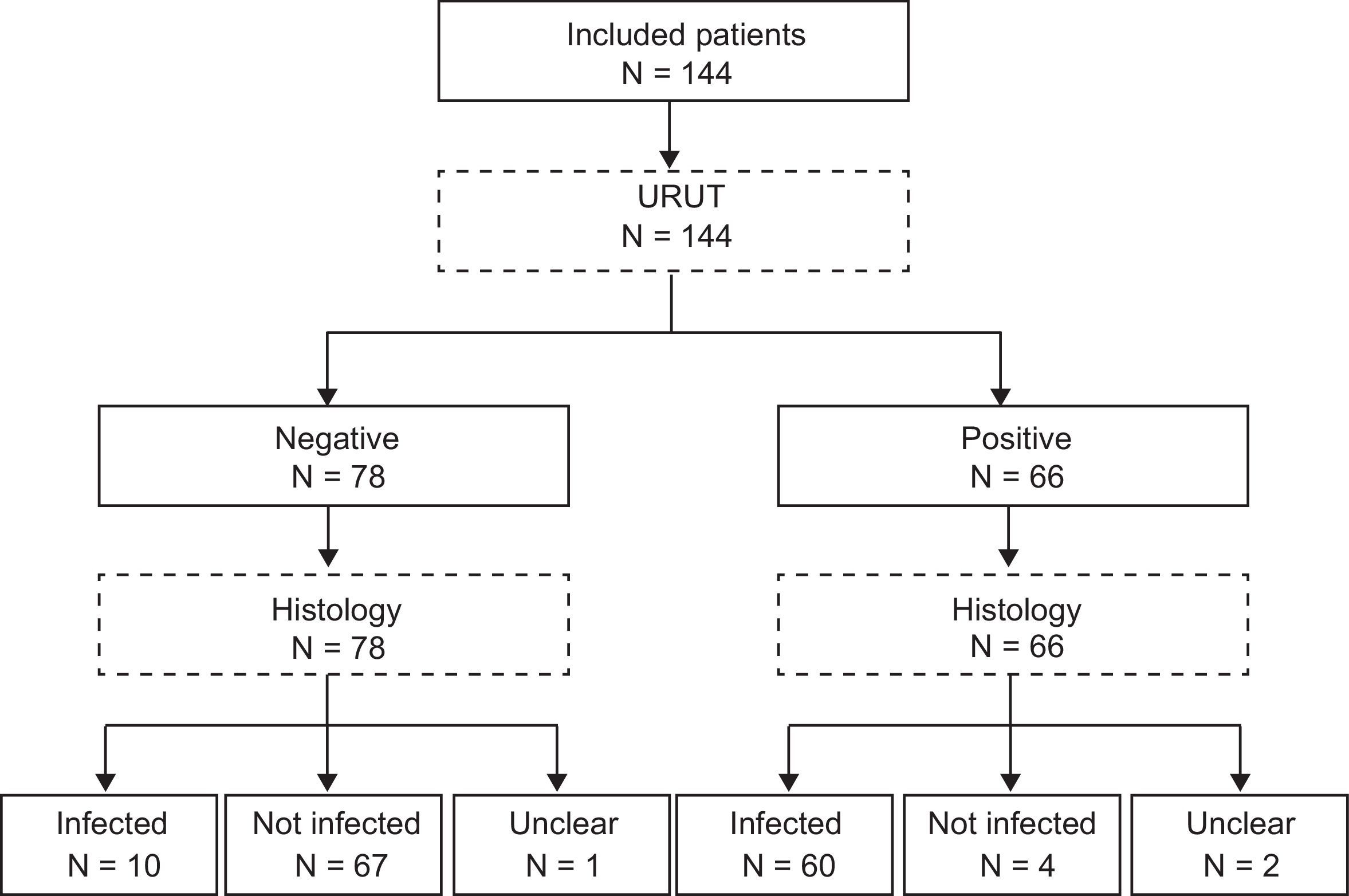
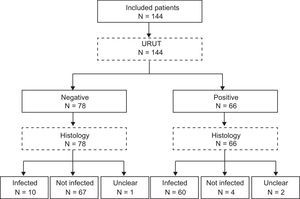
Urease kitsRUT was performed using one out of the three most common kits in Spanish hospitals (CLO test®, Jatrox-test® and Gut plus®). Each hospital used the kit of their own routine clinical practice. The kit was checked out during 24h. Biohit®’s URUT (Biohit®, Helsinki, Finland) was checked 1min, 5min, 30min and 60min after the biopsy was included in the kit's media. Flowchart of the patients is present in Fig. 1 (STARD flowchart).
Ethical issuesThis study was performed with the approval and follow up by the hospitals’ Ethics Committees. The design and development followed the WMA Helsinki Declaration of 1964 and its revisions and all applicable regulations. All patients signed an informed consent.
Statistical analysisMean and standard deviation were calculated for quantitative variables, and percentage and 95% confidence interval (95%CI) for qualitative variables. Sensitivity, specificity, positive and negative predictive values and positive and negative likelihood ratios were calculated for all urease kits. Z-test was used to compare diagnostic success rates. McNemar test was used to compare the distribution of results for both tests. Significance was considered for p<0.05. In order to demonstrate equivalence of RUT and URUT, a sample size of 140 patients was calculated [significance level (alpha) 5%, power (beta) 90% and margin of equivalence (d) 5%].
Results• Study population characteristics
One hundred and forty-four dyspeptic patients were included in 11 hospitals all over Spain in 2009. Average age was 49, and 69% were women. Forty-two percent of patients were taking PPIs. Fifty percent were H. pylori positive by histology. Mean waiting time for positive RUT result was 6.2h.
• Overall diagnostic success of RUT and URUT
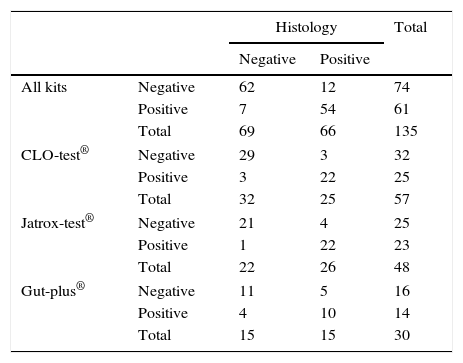
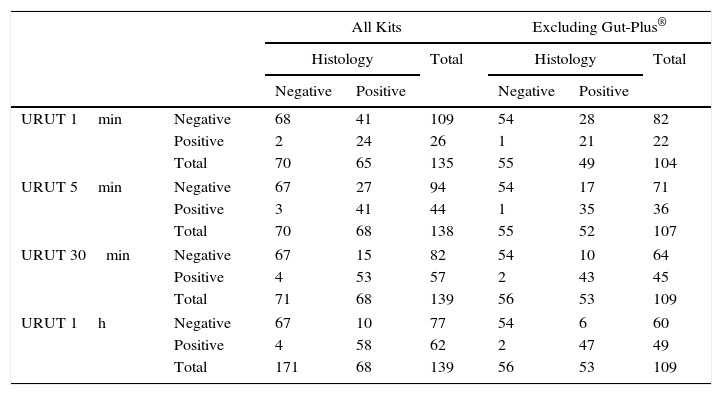
RUT and URUT diagnosis were correct in 85.9% (95%CI=80–92%) (Table 1) and 90.0% (95%CI=85–95%) (Table 2) of the cases respectively.
CrossTable of Rapid Urease Tests (by commercial brand) vs. histology in the diagnosis of H. pylori infection.
| Histology | Total | |||
|---|---|---|---|---|
| Negative | Positive | |||
| All kits | Negative | 62 | 12 | 74 |
| Positive | 7 | 54 | 61 | |
| Total | 69 | 66 | 135 | |
| CLO-test® | Negative | 29 | 3 | 32 |
| Positive | 3 | 22 | 25 | |
| Total | 32 | 25 | 57 | |
| Jatrox-test® | Negative | 21 | 4 | 25 |
| Positive | 1 | 22 | 23 | |
| Total | 22 | 26 | 48 | |
| Gut-plus® | Negative | 11 | 5 | 16 |
| Positive | 4 | 10 | 14 | |
| Total | 15 | 15 | 30 | |
CrossTable of Ultra Rapid Urease Test (URUT) (by time of assessment) vs. histology in the diagnosis of Helicobacter pylori infection.
| All Kits | Excluding Gut-Plus® | ||||||
|---|---|---|---|---|---|---|---|
| Histology | Total | Histology | Total | ||||
| Negative | Positive | Negative | Positive | ||||
| URUT 1min | Negative | 68 | 41 | 109 | 54 | 28 | 82 |
| Positive | 2 | 24 | 26 | 1 | 21 | 22 | |
| Total | 70 | 65 | 135 | 55 | 49 | 104 | |
| URUT 5min | Negative | 67 | 27 | 94 | 54 | 17 | 71 |
| Positive | 3 | 41 | 44 | 1 | 35 | 36 | |
| Total | 70 | 68 | 138 | 55 | 52 | 107 | |
| URUT 30min | Negative | 67 | 15 | 82 | 54 | 10 | 64 |
| Positive | 4 | 53 | 57 | 2 | 43 | 45 | |
| Total | 71 | 68 | 139 | 56 | 53 | 109 | |
| URUT 1h | Negative | 67 | 10 | 77 | 54 | 6 | 60 |
| Positive | 4 | 58 | 62 | 2 | 47 | 49 | |
| Total | 171 | 68 | 139 | 56 | 53 | 109 | |
• RUT by commercial brand
CLO-test®, Jatrox-test® and Gut-plus® were used for the RUT in 37%, 41% and 22% the patients respectively. Comparing their results with the gold standard, CLO-test® and Jatrox-test® each diagnosed correctly 91% of patients but Gut-plus® only 70% (Z-test=2.55, p<0.01). Therefore sub-analyses excluding those patients diagnosed with Gut-plus® were performed (Table 1). Mean time for positive result was 6.2±9.3h (CLO-test® 9.2h; Jatrox-test® 3.0h; Gut-plus® 7.3h).
• URUT by time checked
One minute after biopsies were placed inside the kit's media, URUT correctly diagnosed H. pylori infection in 68% of patients, 78% at 5min, 86% at 30min and 90% at 60min (Table 2).
• Accuracy depending on PPI intake
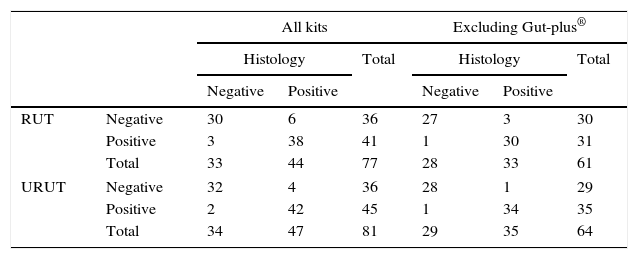
All the previous analyses were performed twice, considering all data and excluding those patients under PPI treatment. RUT and URUT (after 60min) diagnosis was correct in 93% and 97% of patients, including only those patients where the traditional RUT was not Gut-Plus® (Table 3).
CrossTable of Rapid and Ultra Rapid Urease Tests (RUT and URUT) vs. histology in the diagnosis of Helicobacter pylori infection in patients not taking proton pump inhibitor treatment.
| All kits | Excluding Gut-plus® | ||||||
|---|---|---|---|---|---|---|---|
| Histology | Total | Histology | Total | ||||
| Negative | Positive | Negative | Positive | ||||
| RUT | Negative | 30 | 6 | 36 | 27 | 3 | 30 |
| Positive | 3 | 38 | 41 | 1 | 30 | 31 | |
| Total | 33 | 44 | 77 | 28 | 33 | 61 | |
| URUT | Negative | 32 | 4 | 36 | 28 | 1 | 29 |
| Positive | 2 | 42 | 45 | 1 | 34 | 35 | |
| Total | 34 | 47 | 81 | 29 | 35 | 64 | |
• URUT and RUT's accuracy.
Accuracy calculations were performed for RUT and URUT (at checkpoints 30 and 60min). PPI treatment and RUT brand sub-analyses were also studied and data is shown in Table 4. URUT (at 30 and 60min) and RUT's diagnostic successes were not significantly different (Z-test p>0.05). McNemar tests could not demonstrate statistically significant differences when comparing the distribution of success between RUT and URUT.
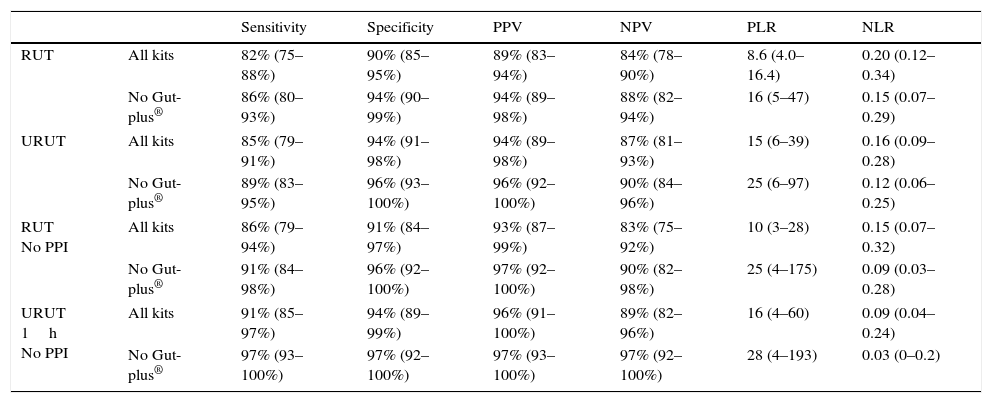
Rapid and Ultra Rapid Urease Test's (RUT and URUT) accuracy for the diagnosis of Helicobacter pylori infection in all subanalyses.
| Sensitivity | Specificity | PPV | NPV | PLR | NLR | ||
|---|---|---|---|---|---|---|---|
| RUT | All kits | 82% (75–88%) | 90% (85–95%) | 89% (83–94%) | 84% (78–90%) | 8.6 (4.0–16.4) | 0.20 (0.12–0.34) |
| No Gut-plus® | 86% (80–93%) | 94% (90–99%) | 94% (89–98%) | 88% (82–94%) | 16 (5–47) | 0.15 (0.07–0.29) | |
| URUT | All kits | 85% (79–91%) | 94% (91–98%) | 94% (89–98%) | 87% (81–93%) | 15 (6–39) | 0.16 (0.09–0.28) |
| No Gut-plus® | 89% (83–95%) | 96% (93–100%) | 96% (92–100%) | 90% (84–96%) | 25 (6–97) | 0.12 (0.06–0.25) | |
| RUT No PPI | All kits | 86% (79–94%) | 91% (84–97%) | 93% (87–99%) | 83% (75–92%) | 10 (3–28) | 0.15 (0.07–0.32) |
| No Gut-plus® | 91% (84–98%) | 96% (92–100%) | 97% (92–100%) | 90% (82–98%) | 25 (4–175) | 0.09 (0.03–0.28) | |
| URUT 1h No PPI | All kits | 91% (85–97%) | 94% (89–99%) | 96% (91–100%) | 89% (82–96%) | 16 (4–60) | 0.09 (0.04–0.24) |
| No Gut-plus® | 97% (93–100%) | 97% (92–100%) | 97% (93–100%) | 97% (92–100%) | 28 (4–193) | 0.03 (0–0.2) | |
PPV: positive predictive value; NPV: negative predictive value; PLR: positive likelihood ratio; NLR: negative likelihood ratio.
95% confidence interval is shown in parentheses.
The present study evaluated the accuracy of RUT and URUT in the diagnosis of H. pylori infection and showed equivalent results for RUT kits (24h result) and Biohit®’s URUT at minutes 30 and 60.
Three RUT kits were studied; Jatrox-test® and CLO-test® offered equivalent results but Gut-plus® had significantly lower accuracy in the studied population. Although this lower accuracy may be due to a low sample size beta error, all the subsequent analyses were calculated both including and excluding patients diagnosed with Gut-plus® kit. Jatrox-test® was able to offer positive results quicker than the other two kits, although the 24h waiting period was still needed in order to avoid false negative results.
The accuracy of Biohit®’s URUT diagnosis at 30min was statistically equivalent to the traditionally used RUT kits. Even though the kit's manual clearly establishes the waiting period in 30min, increasing it to one hour improved sensitivity (better than RUT) in all sub-analyses and did not reduce specificity, thus it may be recommendable to wait 60min before checking the URUT result.
In accordance to previously published data,1,7,8 the intake of PPI drugs the two weeks prior to endoscopy reduced the accuracy of all urease kits in our study. Therefore, the withdrawal of PPIs before endoscopy should be recommended to patients. The results of RUT (Jatrox-test® and CLO-test®) and URUT in our study in patients not taking PPI drugs were excellent, especially in the case of URUT's diagnosis after 60min.
Finally, URUT and RUT kits’ incorrect diagnosis mostly occurred in the same patients and no statistically significant differences were found when comparing the distribution of correct and incorrect diagnosis of both methods. This implies that traditional RUTs limitations regarding diagnostic results,1,4–8 probably due to low bacterial urease activity, are not overcome by URUT.
The main limitation of this study derives from mimicking clinical practice in Spain where there is no systematic implementations of recommendations regarding biopsy collection during endoscopy which did not allow deeper analysis of our patients.
In summary, previous studies comparing diverse ultra rapid urease tests have shown that the accuracy of ultra rapid tests is equivalent to that of traditional ones.10–15 The present study seems to indicate that the URUT version developed by Biohit® is equivalent to the standard RUT used in Spain, reaching approximately 90% sensitivity and specificity in patients naïve to eradication therapy. In general, the use of a validated URUT, as it offers equivalent results than 24h kits, may be recommended in order to start treatment earlier and avoid doubling visits to the outpatient clinic which would reduce overall costs in the management of H. pylori infection and minimize inconveniences to patients.
Conflict of interestsDr. Gisbert has served as speaker, consultant and advisory member for or has received research funding from Almirall, Nycomed, AstraZeneca, Casen Recordati, and Allergan. Dr. McNicholl has received retribution from Allergan for formative actions. Dr. Castro-Fernandez has received retribution from Allergan for formative actions. Dr. Perez-Aísa has received retribution from Allergan and Mylan for formative actions.
CIBERehd is funded by the Instituto de Salud Carlos III. This study was not funded by any Pharmaceutical Company.
| Section and Topic | Item | On page | |
|---|---|---|---|
| TITLE/ABSTRACT/ KEYWORDS | 1 | Identify the article as a study of diagnostic accuracy (recommend MeSH heading ‘sensitivity and specificity’). | 1 |
| INTRODUCTION | 2 | State the research questions or study aims, such as estimating diagnostic accuracy or comparing accuracy between tests or across participant groups. | 2 |
| METHODS | |||
| Participants | 3 | The study population: The inclusion and exclusion criteria, setting and locations where data were collected. | 7 |
| 4 | Participant recruitment: Was recruitment based on presenting symptoms, results from previous tests, or the fact that the participants had received the index tests or the reference standard? | 7 | |
| 5 | Participant sampling: Was the study population a consecutive series of participants defined by the selection criteria in item 3 and 4? If not, specify how participants were further selected. | 7 | |
| 6 | Data collection: Was data collection planned before the index test and reference standard were performed (prospective study) or after (retrospective study)? | 7 | |
| Test methods | 7 | The reference standard and its rationale. | |
| 8 | Technical specifications of material and methods involved including how and when measurements were taken, and/or cite references for index tests and reference standard. | 7 | |
| 9 | Definition of and rationale for the units, cut-offs and/or categories of the results of the index tests and the reference standard. | 7-8 | |
| 10 | The number, training and expertise of the persons executing and reading the index tests and the reference standard. | 7 | |
| 11 | Whether or not the readers of the index tests and reference standard were blind (masked) to the results of the other test and describe any other clinical information available to the readers. | 7 | |
| Statistical methods | 12 | Methods for calculating or comparing measures of diagnostic accuracy, and the statistical methods used to quantify uncertainty (e.g. 95% confidence intervals). | 8 |
| 13 | Methods for calculating test reproducibility, if done. | ||
| RESULTS | |||
| Participants | 14 | When study was performed, including beginning and end dates of recruitment. | 9 |
| 15 | Clinical and demographic characteristics of the study population (at least information on age, gender, spectrum of presenting symptoms). | 9 | |
| 16 | The number of participants satisfying the criteria for inclusion who did or did not undergo the index tests and/or the reference standard; describe why participants failed to undergo either test (a flow diagram is strongly recommended). | 9 & 15 | |
| Test results | 17 | Time-interval between the index tests and the reference standard, and any treatment administered in between. | 9 |
| 18 | Distribution of severity of disease (define criteria) in those with the target condition; other diagnoses in participants without the target condition. | N/A | |
| 19 | A cross tabulation of the results of the index tests (including indeterminate and missing results) by the results of the reference standard; for continuous results, the distribution of the test results by the results of the reference standard. | 16-19 | |
| 20 | Any adverse events from performing the index tests or the reference standard. | N/A | |
| Estimates | 21 | Estimates of diagnostic accuracy and measures of statistical uncertainty (e.g. 95% confidence intervals). | 16-19 |
| 22 | How indeterminate results, missing data and outliers of the index tests were handled. | N/A | |
| 23 | Estimates of variability of diagnostic accuracy between subgroups of participants, readers or centers, if done. | 9 | |
| 24 | Estimates of test reproducibility, if done. | N/A | |
| DISCUSSION | 25 | Discuss the clinical applicability of the study findings. | 11-12 |