To evaluate effectiveness and safety of adalimumab in CD patients of the Madrid area and identify predictors of response.
MethodsMulticenter retrospective survey of all CD patients treated with adalimumab in 9 hospitals of the Madrid area (Spain). Univariate and multivariate analysis of predictors of response was performed.
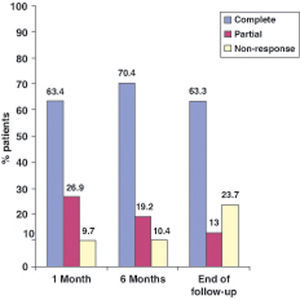
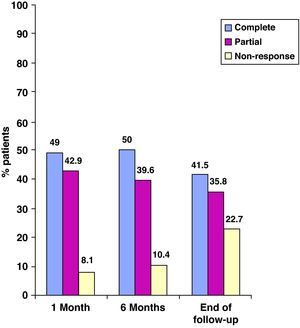
Results174 patients included (50% males) with a median follow-up of 40 weeks. 30% had active perianal fistulizing disease at the beginning of the therapy with adalimumab. 59% had been previously treated with infliximab, being the lost of response (42.2%) the most frequent cause of withdrawal of the drug. 33% of patients needed dose escalation from every-other week to everyweek. The median time for this dose escalation was 33 weeks (range 2-120). The percentages of complete response at 4 weeks, 6 months and end of follow-up were 63, 70 and 63% in luminal disease and 49, 50 and 41% in perianal disease respectively. The prevalence of adverse events was 18% (most frequent was: 5 abscesses) causing the withdrawal of the drug in 21% of them.
ConclusionsAdalimumab is effective and safe for the management of CD, even in refractory cases to infliximab.
Evaluar la eficacia y seguridad de adalimumab en pacientes con enfermedad de Crohn (EC) de la comunidad de Madrid e identificar los factores predictivos de respuesta.
MétodosEstudio multicéntrico retrospectivo de todos los pacientes con EC tratados con adalimumab en 9 hospitales de la Comunidad de Madrid (España). Se llevó a cabo análisis univariado y multivariado de predictores de respuesta.
Resultados174 pacientes incluidos (50% varones) con una mediana de seguimiento de 40 semanas. El 30% tenía enfermedad fistulosa perianal activa al inicio de la terapia con adalimumab. El 59% habían sido tratados previamente con infliximab, siendo la pérdida de respuesta (42,2%) la causa más frecuente de la retirada del fármaco. Un 33% de los pacientes necesitaron aumentar la dosis de todos los demás cada semana. La mediana del tiempo para este aumento de la dosis fue de 33 semanas (rango 2-120). Los porcentajes de respuesta completa a las 4 semanas, 6 meses y al final del seguimiento fueron de 63, 70 y 63% en la enfermedad luminal y 49, 50 y 41% en la enfermedad perianal, respectivamente. La prevalencia de eventos adversos fue de 18% (más frecuente: 5 abscesos) provocando la retirada del fármaco en el 21% de ellos.
ConclusionesEl adalimumab es eficaz y seguro en el tratamiento de la EC, incluso en los casos refractarios a infliximab.
The use of tumor necrosis factor (TNF) antagonists has changed the therapeutic approach to Crohn's disease (CD) in the last decade, especially in patients who are refractory to conventional therapy or have severe presentations of the disease.1
Infliximab (IFX) is a chimeric anti-TNF IgG1 monoclonal antibody that has been shown to induce and maintain clinical response in CD characterized by both active inflammatory and fistulizing disease.2 Nevertheless, during long-term treatment, a significant proportion of patients suffer side effects or a loss of response. In this sense, Gisbert et al3 estimated an annual risk for loss of efficacy of 13% per patient-year. The development of anti-infliximab antibodies or the intervention of still undefined immunological mechanisms may explain these situations.4
Adalimumab (ADA) is a fully human anti-TNF IgG1 monoclonal antibody that has also been shown to induce and maintain clinical response in active CD not controlled by corticosteroids, inmunosuppresants or both.2 This drug has been used in Spain since the summer of 2005 –initially on a compassionate use basis in patients previously responsive to IFX but who suffered secondary events or a loss of response,3 and since June 2007 as approved therapy in CD. Although several clinical trials5–10 and a metaanalysis2 have demonstrated both the efficacy and safety of ADA in CD, we believe it to be of great interest to determine whether such efficacy and safety are also present when ADA is used in real clinical practice.
The main aim of this study was to evaluate the effectiveness and safety of ADA in CD patients in the area of Madrid (Spain), while the secondary aim was to identify predictors of response.
MethodsThis was a multicenter, retrospective, open-label, observational study. To evaluate our experience with ADA, we gathered patients with CD from 9 hospitals in the area of Madrid (the diagnosis of CD being based on the established clinical, endoscopic, radiological and histological criteria) who had been treated with at least two doses of this drug since its approval in the summer of 2005. The corresponding data were collected from June to August 2009.
The study variables included demographic data, features of CD (duration, extent of the disease, severity and previous treatments –both medical and surgical–, data from the previous IFX treatment in those who had received the drug (duration, regimen used, response and cause of discontinuation), and finally data from ADA therapy (effectiveness and safety at one month, 6 months and at the end of follow-up). In evaluating effectiveness, we distinguished luminal from perianal fistulizing disease.
In luminal inflammatory disease complete response was defined as the disappearance of symptoms according to the clinical evaluation of the supervising physician and normalization of all inflammation parameters without the need of glucocorticoids therapy. In contrast, partial response was defined as a significant improvement in symptoms and inflammation parameters, but without full resolution.
In perianal fistulizing disease, complete response was defined as the closure of all fistulas. On the other hand, partial response was defined as the closure of at least 50% of all fistulas.
In those patients previously treated with IFX, we registered the reasons for discontinuation of the drug, classified into 6 different groups:
- –
Primary non-responders: defined as the absence of response to IFX after the three doses of induction therapy at 0, 2 and 6 weeks.
- –
Loss of response: patients in this group had an initial response to IFX but worsened during follow-up despite reduction of the interval of infusions or the administration of higher doses
- –
Secondary events: this group included patients who had suffered an infusion reaction or other type of adverse event to IFX causing withdrawal of the drug.
- –
Long drug holiday: patients who received only the induction regimen according to the scientific evidence of the period when this drug was administered.
- –
Patient's preference: this group included patients who despite a response to IFX decided to change to ADA due to comfort reasons (easy administration at home).
- –
Clinical improvement: in this group IFX was withdrawn due to quiescent disease.
- –
Quantitative variables were summarized as their mean and standard deviation (SD), or median and range or interquartile range (IQR) for variables without a normal distribution. Qualitative variables were summarized as percentages and 95% IC. Univariate and multivariate analyses of predictors of response were performed and logistic regression was performed when indicated. The different variables analysed are shown in Table 1.
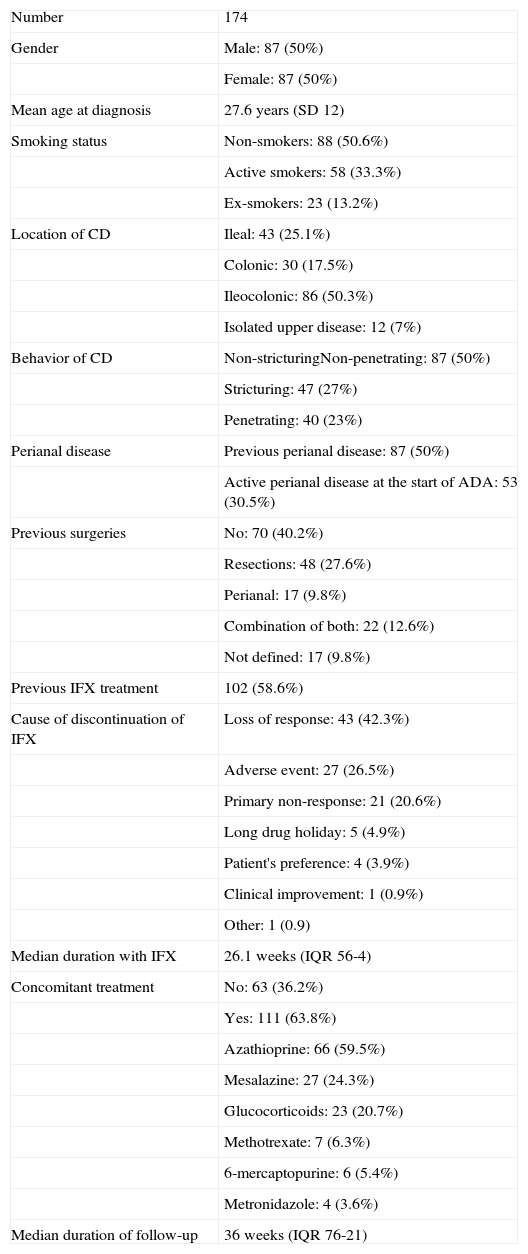
Table 1.Clinical and demographics features of Crohn's disease.
Number 174 Gender Male: 87 (50%) Female: 87 (50%) Mean age at diagnosis 27.6 years (SD 12) Smoking status Non-smokers: 88 (50.6%) Active smokers: 58 (33.3%) Ex-smokers: 23 (13.2%) Location of CD Ileal: 43 (25.1%) Colonic: 30 (17.5%) Ileocolonic: 86 (50.3%) Isolated upper disease: 12 (7%) Behavior of CD Non-stricturingNon-penetrating: 87 (50%) Stricturing: 47 (27%) Penetrating: 40 (23%) Perianal disease Previous perianal disease: 87 (50%) Active perianal disease at the start of ADA: 53 (30.5%) Previous surgeries No: 70 (40.2%) Resections: 48 (27.6%) Perianal: 17 (9.8%) Combination of both: 22 (12.6%) Not defined: 17 (9.8%) Previous IFX treatment 102 (58.6%) Cause of discontinuation of IFX Loss of response: 43 (42.3%) Adverse event: 27 (26.5%) Primary non-response: 21 (20.6%) Long drug holiday: 5 (4.9%) Patient's preference: 4 (3.9%) Clinical improvement: 1 (0.9%) Other: 1 (0.9) Median duration with IFX 26.1 weeks (IQR 56-4) Concomitant treatment No: 63 (36.2%) Yes: 111 (63.8%) Azathioprine: 66 (59.5%) Mesalazine: 27 (24.3%) Glucocorticoids: 23 (20.7%) Methotrexate: 7 (6.3%) 6-mercaptopurine: 6 (5.4%) Metronidazole: 4 (3.6%) Median duration of follow-up 36 weeks (IQR 76-21)
A total of 174 patients were included, with a balanced gender distribution (50% males). The mean age at the start of ADA treatment was 28 years (SD 12). The median duration of follow-up was 36 weeks (IQR 21-76). As regards smoking, 88 patients (50.6%) had never smoked, 58 (33.3%) were active smokers, and 23 (13.2%) had quitted smoking.
Features of Crohn's diseaseThe baseline clinical and demographic characteristics of the patients are shown in Table 1. The mean age at diagnosis of CD was 27.6 years (SD 12). The most frequent distribution and behavior of the disease were ileocolic (50%) and inflammatory (50%), respectively. Out of the 174 patients, 87 (50%) had developed perianal fistulizing disease, and among them 53 (30.5%) had active draining fistulas at the beginning of the study. A total of 104 subjects (59.8%) had undergone previous surgeries related to CD; of these, 48 (27.6%) corresponded to resections, 17 (9.8%) to perianal surgery, 22 (12.6%) to a combination of both, and in 17 subjects (9.8%) we could not establish the type of surgery performed. Regarding the previous administration of IFX, 102 patients (58.6%) had been treated with this drug before starting ADA. The most frequent cause of IFX discontinuation was a loss of response, in a total of 43 patients (35.2%). The median duration of IFX therapy was 26.1 weeks (IQR 56-4). Lastly, 111 patients (63.8%) were receiving concomitant treatment - the most frequent being azathioprine (59.5% of all patients). The most frequent indication for initiating an anti-TNF drug was luminal disease (69.5%), followed by perianal disease (19%) and a combination of both (11.5%). ADA therapy was started as second line treatment after the administration of IFX in 61% of the patients. As already commented, the most common cause of withdrawal was a loss of response (35.2%). The great majority of patients (93.7%) received the ADA induction regimen with 160 and 80mg at weeks 0 and 2, respectively. Maintenance treatment consisted of the administration of 40mg every two weeks or weekly, depending on the need for dose escalation due to a loss of response during follow-up. In this sense, 57 patients (32.8%) required dose escalation, the median time to dose escalation being 33 weeks (range 2-120).
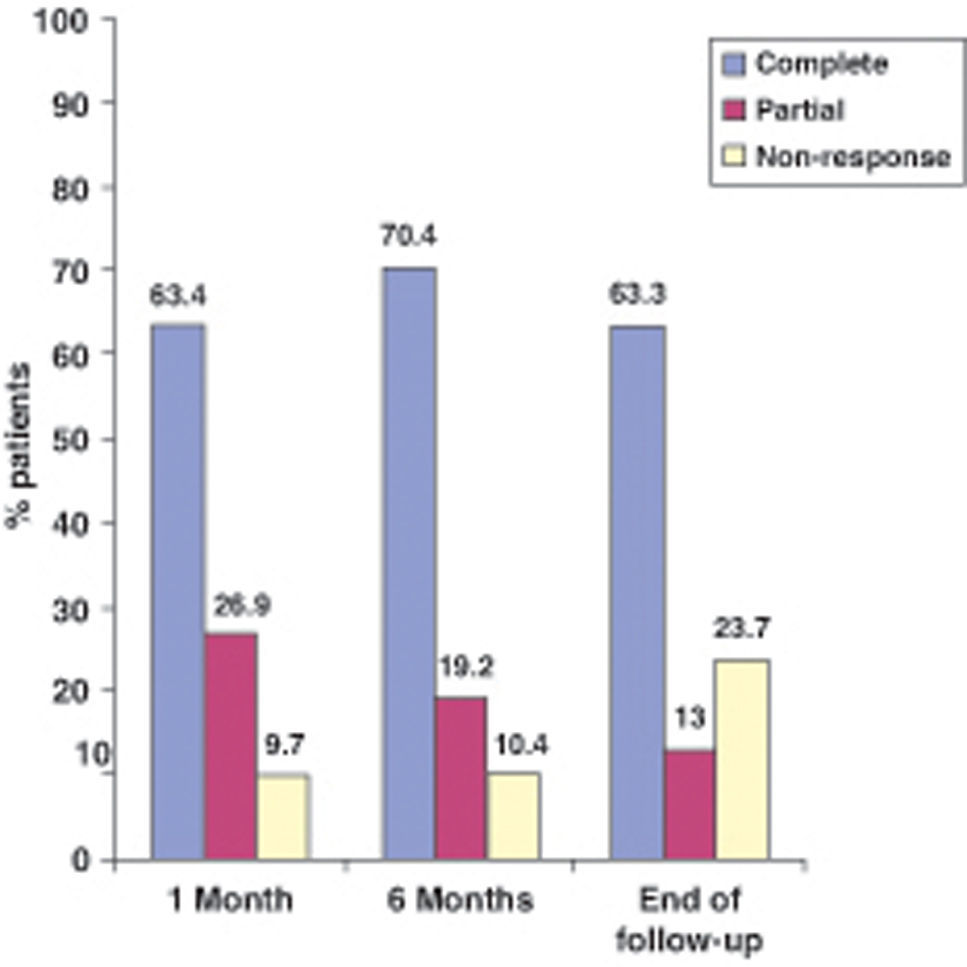
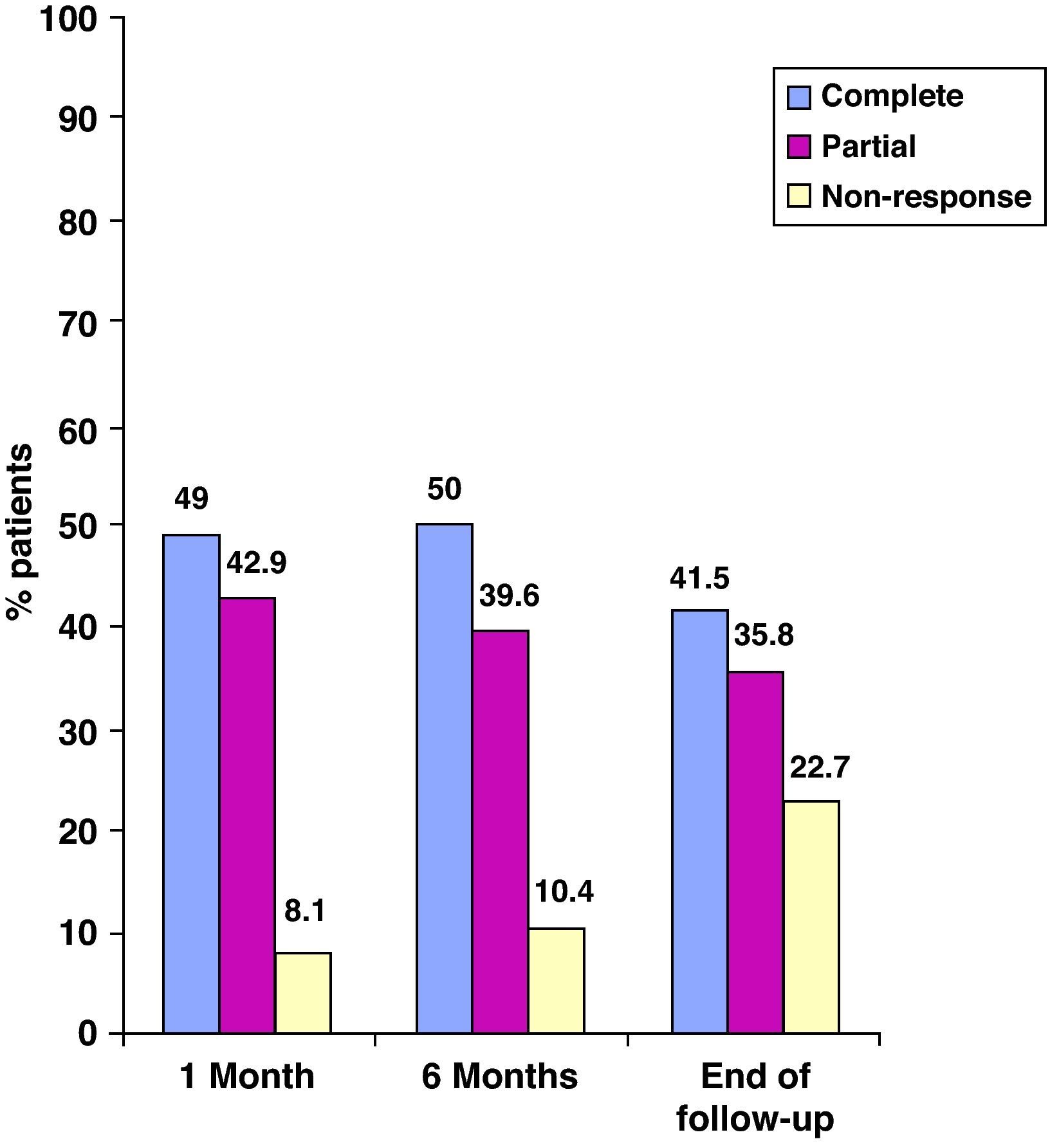
EfectivenessIn evaluating the effectiveness of ADA, we distinguished luminal from perianal fistulizing disease (Figures 1 and 2). In the former, the percentages of complete response at one month, 6 months and at the end of follow-up were 63.4%, 70.4% and 63.3%, respectively, whereas in the latter the percentages were 49%, 50% and 41.5%, respectively.
Regarding predictors of response, we performed uni- and multivariate analyses of the different study variables. In this context, we only found that those who needed dose escalation showed a poorer response (p=0.017). It should be noted that we did not find any significant difference in effectiveness between those who received adalimumab as first line treatment and those who had previously received infliximab (50 and 56.3% of complete response at the end of follow up in luminal disease, p=0.829; and 64 and 33.3% in perianal fistulizing disease, p=0.164). Likewise, we found no difference in the effectiveness of ADA according to the cause of suspension of IFX in those cases in which this drug was administrated in first place (primary non-response versus other causes).
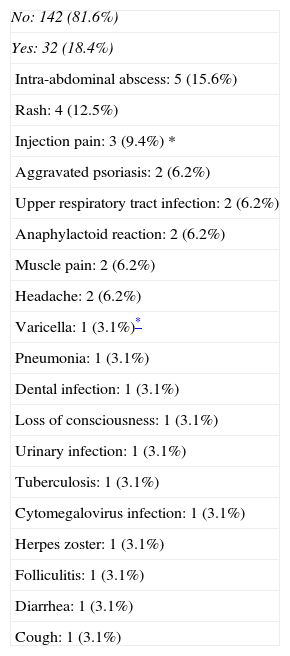
SafetyOf the 174 patients included in our study, 32 (18.4%) suffered adverse events, and most of these were mild (Table 2). The most common adverse event was the development of intra-abdominal abscesses (2.9%). Seven patients (21% of all adverse events) developed serious adverse events requiring drug discontinuation. Three of these cases corresponded to infectious complications (tuberculosis, cytomegalovirus infection and intra-abdominal abscess). In another two cases suspension was required due to an anaphylactic reaction, while in the remaining two treatment discontinuation was decided due to an exacerbation of psoriasis and loss of consciousness of unknown origin, coinciding with the administration of ADA.
Summary of adverse events.
| No: 142 (81.6%) |
| Yes: 32 (18.4%) |
| Intra-abdominal abscess: 5 (15.6%) |
| Rash: 4 (12.5%) |
| Injection pain: 3 (9.4%) * |
| Aggravated psoriasis: 2 (6.2%) |
| Upper respiratory tract infection: 2 (6.2%) |
| Anaphylactoid reaction: 2 (6.2%) |
| Muscle pain: 2 (6.2%) |
| Headache: 2 (6.2%) |
| Varicella: 1 (3.1%)* |
| Pneumonia: 1 (3.1%) |
| Dental infection: 1 (3.1%) |
| Loss of consciousness: 1 (3.1%) |
| Urinary infection: 1 (3.1%) |
| Tuberculosis: 1 (3.1%) |
| Cytomegalovirus infection: 1 (3.1%) |
| Herpes zoster: 1 (3.1%) |
| Folliculitis: 1 (3.1%) |
| Diarrhea: 1 (3.1%) |
| Cough: 1 (3.1%) |
Medical therapy for CD has greatly improved since the introduction of anti-TNF drugs.1 IFX was the first of these molecules introduced in the treatment of CD in August 1998 in USA and August 1999 in Europe and has been shown to be effective in inducing and maintaining remission of the disease in patients with both luminal and perianal disease.2 Nonetheless, a significant proportion of patients can suffer adverse events or a loss of response. These events could be explained, at least partially, by the development of antibodies against IFX or the intervention of other still undefined immunological mechanisms.4
These problems underscore the need for the development of new drugs capable of resolving some of these issues. ADA was introduced in this context. It is a fully human anti-TNF IgG1 monoclonal antibody administered via the subcutaneous route. Although it was believed that ADA would diminish the production of antibodies due to its human nature, the presence of such immunoglobulins has been reported in two clinical trials.5,7 At present, a large body of data is being produced by clinical trials,5–10 metaanalyses2 and non-controlled studies,11–16 demonstrating the effectiveness of ADA in inducing and maintaining clinical response and remission.
The first evidence of its usefulness in CD came from non-controlled studies (11-15). This in turn was followed by data from randomized controlled studies. The CLASSIC-I (Clinical assessment of adalimumab safety and efficacy studied as induction therapy in Crohn's disease) was the first of these trials. It was a four-week randomized controlled induction study showing ADA to be effective in inducing disease remission in patients with moderately to severely active CD who were naïve to anti-TNF therapy (36% in the group treated with 160/80mg of ADA at weeks 0 and 2 versus 12% in the placebo group; p=0.001). Three induction doses were compared (160/80, 80/40 and 40/20mg in weeks 0 and 2). The highest dose regimen (160/80mg) showed the greatest efficacy and was the only one to achieve statistical significance.5 Subsequently, the CLASSIC-II7 and CHARM6 trials, with a median follow-up of 56 weeks, showed that ADA is also effective in maintaining disease remission over the long term when compared with placebo (79-82% and 44% in the CLASSIC-II trial, and 41-36% and 12% in the CHARM trial, in the ADA and placebo groups, respectively). There was no significant difference in efficacy between the two regimens studied (40mg weekly versus every two weeks). Therefore, the former regimen was established as the more convenient option, enabling dose escalation in those patients showing a loss of response.
Likewise, it was essential to establish the efficacy of ADA in patients previously treated with IFX. Preliminary data from uncontrolled studies11,13–15 showed its possible utility in this context. Accordingly, a randomized controlled study was carried out in order to address this issue.8 Its results showed ADA to be effective when compared with placebo in this subgroup of patients (21% and 7% in the ADA and placebo group, respectively; p<0.001). Two later uncontrolled studies supported these results.12,16
Lastly, it was also very important to establish the efficacy of this drug in patients with perianal fistulizing CD. The first data were contradictory. On one hand, the CLASSIC I5 and GAIN8 trials did not show an improvement in this subgroup of patients when compared with placebo. On the other hand, in a sub-analysis of the CHARM trial,6 complete closure of the draining fistulas was observed in 30% and 13% of the patients treated with ADA and placebo, respectively (p<0.05). At the same time, results from uncontrolled studies supported the efficacy of ADA in this patient subgroup11,12,16. In order to clarify these conflicting results, a randomized controlled trial was designed10 showing ADA to be more effective than placebo both in inducing (60% at two years) and in maintaining closure of the draining fistulas (90% at two years).
The present study aims to evaluate both the effectiveness and safety of ADA in real life clinical practice. To this effect, we documented the experience with ADA in 9 hospitals in the area of Madrid since approval of the drug in the summer of 2005. Similar studies have been made in the last two years in Spain,16, Scotland17,18 and the United States19, but our study is by far the largest observational cohort study evaluating efficacy and safety of adalimumab in CD in real life (174 versus 98 in Ho's study18). Our results confirm that ADA is effective in inducing and maintaining remission in both luminal and perianal fistulizing disease. Accordingly, in the former presentation of the disease we recorded complete response rates of 63.4%, 70.4% and 63.3% at one month, 6 months and at the end of follow-up, respectively. These results are similar to those obtained in other studies,11,12,17,18 though at the same time the response rates, particularly at four weeks, are significantly higher than those reported by other authors.5,8,16,19. This difference in effectiveness could be partially explained by the retrospective nature of our study and the heterogeneity in both the population studied and in the criteria chosen for defining response to therapy in the different trials. Thus, in some of these studies efficacy is evaluated based on different scores (CDAI score or the Harvey-Bradshaw index) whereas in others (including our own study) efficacy is based on clinical evaluation of the corresponding physician.
In perianal fistulizing disease, ADA was less effective, with complete response rates of 49%, 50% and 41.5% at one month, 6 months and at the end of follow-up, respectively. Once again, these percentages are similar to those of some other authors,10,12 but significantly higher than those obtained in other trials, especially at four weeks.11,16. These differences could be explained by the same reasons described above.
Up to 32.8% of all patients required dose escalation due to a loss of response. In such cases, the median time to dose escalation was 33 weeks (range 2-120). In the multivariate analysis, the only variable showing a significant association to poorer ADA therapy response was this need for dose escalation. This seems reasonable, because these patients represent the most refractory disease subgroup. Finally, although we did not find any significant difference in effectiveness between those who received adalimumab as first line treatment and those who had previously received infliximab (50 and 56.3% of complete response at the end of follow up in luminal disease, p=0.829; and 64 and 33.3% in perianal fistulizing disease, p=0.164), we realised that in fistulizing disease, those who received ADA as first line treatment responded much better at the end of follow-up, possibly reflecting an underpowered result due to paucity of patients in this subgroup.
Regarding safety, 18.4% of the patients suffered adverse events. Although most of these were mild, severe adverse effects were described such as 5 abscesses and 1 case of tuberculosis (TBC) despite following the prevention guidelines of TBC developed by GETTECCU. This percentage is much lower than in other non-controlled studies,12,16–18 in which adverse events rates of up to 54% have been reported. These differences can also be explained by the heterogeneity of the population studied and the retrospective nature of our study. In any case, these results support the safety of ADA in the real clinical practice setting and at the same time underscore the need for a close follow-up in these patients due to the possibility of the development of severe adverse effects.
Lastly, some limitations of our study should be commented. Firstly, data collection was carried out retrospectively, which makes it difficult to establish a more detailed and homogeneous evaluation of the patients. Secondly, our results cannot be directly compared with those of most other clinical trials or non-controlled studies, because the main aims and variables of response differ. Finally, these results reflect the experience gained in the management of CD patients treated with adalimumab in a group of hospitals within the same region. We therefore must be cautious in attempting to extrapolate these results to other populations.
In conclusion, our results demonstrate both the effectiveness and safety of ADA in real clinical practice. Future studies should serve to define response predicting factors allowing us to select the most appropriate therapy for each patient.
Conflict of interestThe authors declare there are not conflicts of interest.