Strong acid inhibition increases cure rates with triple therapy and 14-day are more effective than 7-day treatments. The combination of amoxicillin plus metronidazole at full doses has been shown to overcome metronidazole resistance and to achieve good eradication rates even in patients harboring resistant strains. No previous studies have been reported in Latin-America with this optimized triple-therapy scheme.
AimsThe aim of the present study was to assess the eradication rate and tolerance of a new first-line treatment regimen associating strong acid inhibition, amoxicillin and metronidazole.
MethodsPatients from the Clínica de Gastroenterología of the Hospital de Clínicas (Montevideo, Uruguay) were included. Hp status was mainly assessed by at least one of the following: histologyor urea breath test (UBT). A 14-day treatment was prescribed comprising esomeprazole 40mg twice a day plus amoxicillin 1g and metronidazole 500mg, both three times a day. H. pylori cure was assessed by UBT.
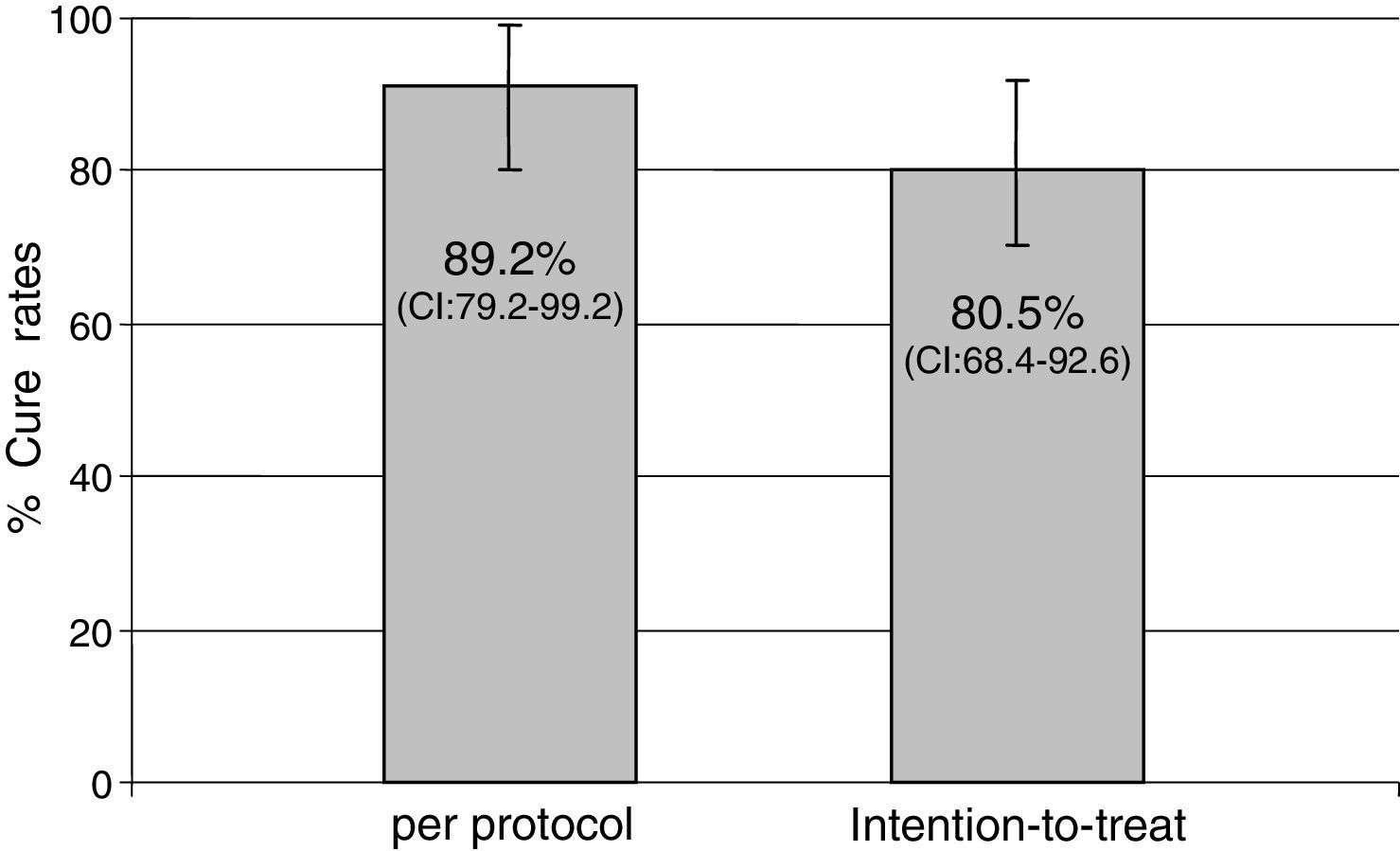
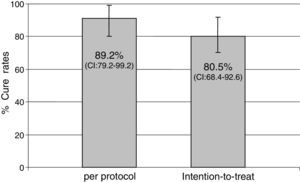
ResultsForty-one patients were enrolled. Mean age was 53.3±13 years and 17.1% of patients were male. Main indications for treatment were: functional dyspepsia (27.5%), gastritis (45%), gastric or duodenal erosions (20%), gastric ulcer (5%) and intestinal metaplasia (2.5%). H. pylori eradication was achieved in 33 of the 37 patients who returned for follow-up. Eradication rates were 80.5% (95% CI: 68.4–92.6) by intention-to-treat (ITT) analysis and 89.2% (95% CI; 79.2–99.2) per protocol (PP). No major side effects were reported; 26 patients (65.8%) complained of mild side effects (nausea, diarrhea and headache).
ConclusionsCure rates of this triple therapy including esomeprazole, amoxicillin and metronidazole were 81% per ITT and the treatment was well tolerated. These optimal results with a simple clarithromycin-free triple therapy are better than described for standard triple therapy but there is still room for improvement to reach the desired target of 90% per ITT.
La inhibición ácida potente aumenta las tasas de curación de la triple terapia, y 14 días de tratamiento son más efectivos que 7 días. La combinación de amoxicilina y metronidazol a dosis completas ha demostrado que supera la resistencia al metronidazol y que consigue buenas tasas de erradicación, incluso en pacientes que poseen cepas resistentes. No se han reportado estudios con este tratamiento triple optimizado en Latinoamérica.
ObjetivosEl objetivo del presente estudio es valorar las tasas de erradicación y la tolerancia de un nuevo tratamiento de primera línea que asocia inhibición ácida potente, amoxicilina y metronidazol.
MétodosPacientes provenientes de la Clínica de Gastroenterología del Hospital de Clínicas (Montevideo, Uruguay) se incluyeron en el estudio. La infección por Helicobacter pylori fue demostrada por, al menos, una de las siguientes: histología o test del aliento. Se prescribió un tratamiento de 14 días con esomeprazol 40mg 2 veces al día junto a amoxicilina 1g y metronidazol 500mg ambos 3 veces al día. La curación de Helicobacter pylori fue confirmada por el test del aliento.
ResultadosSe incluyeron 41 pacientes. La edad media fue de 53,3±13 años y el 17,1% de los pacientes eran hombres. La principal indicación para el tratamiento fue: dispepsia funcional (27,5%); gastritis (45%), erosiones gástricas o duodenales (20%), úlcera gástrica (5%) y metaplasia intestinal (2,5%). La erradicación de Helicobacter pylori se consiguió en 33 de los 37 pacientes que regresaron para el test de control. Las tasas de erradicación fueron del 80,5% (IC 95%: 68,4-92,6) por intención de tratar y del 89,2% (IC 95%: 79,2-99,2) por protocolo. No se reportaron efectos secundarios graves. Veintiséis pacientes (65,8%) presentaron efectos secundarios leves (náuseas, diarrea y cefalea).
ConclusionesLas tasas de erradicación de esta triple terapia que incluye esomeprazol, amoxicilina y metronidazol fueron del 81% por intención de tratar y el tratamiento fue bien tolerado. Estos resultados óptimos con un tratamiento triple sencillo, sin claritromicina, son mejores que los descritos para el tratamiento triple estándar, pero todavía existe espacio de mejora para alcanzar el objetivo deseado del 90% por intención de tratar.
Many studies have revealed a strong relation between the Helicobacter pylori infection and gastric disorders, especially functional dyspepsia, peptic ulcer disease, gastric carcinoma and mucosa associated lymphoid tissue-lymphoma.1 Moreover, extra-digestive diseases are also associated with H. pylori; idiopathic thrombocytopenic purpura and idiopathic iron deficiency anemia.2 Therefore, eradication of H. pylori is an important issue, which still remains unsolved.
Today, there is still not a single optimal antibiotic treatment for eradication. Almost all of the evidence supporting different therapeutic regimens come from Europe and Asia; there are scant data from Latin America, a region with some of the world's highest gastric cancer mortality rates.3,4
For many years, triple therapy combining a proton pump inhibitor (PPI), amoxicillin and clarithromycin for 7–10 days has been the undisputed choice for first-line therapy worldwide5,6 and is the most used and recommended regimen in South America.7,8
In recent years, however, many studies worldwide have found unacceptably low cure rates for this “classical” or “legacy” triple therapy.9,10 As clarithromycin resistance is the strongest predictor of treatment failure, cure rates are likely to fall further as antimicrobial resistance becomes more prevalent worldwide.
Alternatives to triple therapy, however, have not been definitively established. The current recommendations for first-line therapy in areas where triple therapy fails are either classical, quadruple therapy containing bismuth or quadruple therapies containing clarithromycin. Although superior to triple therapy, these approaches have clear shortcomings. “Classical” bismuth-containing quadruple therapy combines a proton pump inhibitor (PPI) twice a day, metronidazole three times a day and tetracycline and bismuth four times a day for 10–14 days: a complicated schedule requiring a large number of daily pills. A single-pill compound combining tetracycline, metronidazole and bismuth given every 6h has been shown to be superior to a 7-day standard triple therapy in a randomized, open label, control trial in 39 sites in Europe. However, although this single-pill compound probably facilitates adherence to treatment, the ITT cure rate was only 80%.11 Thereby, classical bismuth-based quadruple therapy have been recommended as second-line therapy by the Maastricht V Consensus Report6 and other international clinical guidelines.12 We have no information about its efficacy in other populations including Latin America.
Latin American Consensus states that standard triple therapy for 14 days is comparable to sequential therapy as empiric therapy for H. pylori infection in diverse Latin American populations. Sequential therapy is probably a better first-line alternative regimen in areas with high prevalence of clarithromycin-resistant strains.13 Concomitant quadruple therapy for 10 or 14 days should be studied in Latin America and may be a good first or second-line alternative in areas with high prevalence of dual resistance to clarithromycin and metronidazole.
Regarding treatment regimens, the most important Latin American study is a recent multicenter RCT comparing 14-day triple therapy vs. 5-day concomitant (lansoprazole, amoxicillin, clarithromycin and metronidazole) and 10-day sequential treatment in seven sites (Chile, Colombia, Costa Rica, Honduras, Nicaragua, and Mexico), including 1463 participants.9 The ITT eradication rate with triple therapy and sequential therapy was similar (82.2% and 76.5%, respectively, p=NS). Greenberg and colleagues report that traditional triple therapy was the best of the three clarithromycin containing regimens tested. However, they did not identify a reliably effective regimen overall and even their best regimen provided unacceptably low treatment results at four of the seven sites. An updated evaluation showed that the estimated eradication success rate after 1 year of follow-up was virtually the same for both triple therapy and sequential treatment (80.4% and 79.8%, respectively).14
As current regimens just approach 80% cure rates, more efficacious treatment schemes are clearly needed. In this context using a triple therapy with high doses of a PPI plus amoxicillin and metronidazole in a pharmacologically adequate schedule may be a useful alternative. The choice of amoxicillin and metronidazole in a setting of increasing antibiotic resistance is reasonable: resistance to amoxicillin is extremely unusual, due to the need for more than one mutation in the bacteria genome.15,16 Previous studies have also demonstrated that metronidazole resistance can be overcome using high doses of this antibiotic for 10 days or more17 and extending triple treatment duration to 14 days also increases cure rates.18,19
In addition to using an antibiotic combination that is efficacious in the setting of increasing resistances, a second useful measure for raising cure rates of H. pylori treatment is to increase the level of acid suppression. Maximum increase in eradication was observed with very strong acid inhibition: that is, when the PPI used was esomeprazole and this drug was given at a 40mg dose twice a day.20
The aim of the present study was, thus, to evaluate with a pilot trial in Uruguay, the efficacy of a triple schedule combining esomeprazole 40mg twice a day plus amoxicillin 1g and metronidazole 500mg three times a day, all given with meals over a 14-day period, as an empirical first-line approach for curing H. pylori infection.
MethodsPatients with indication for H. pylori eradication from the Clínica de Gastroenterología of the Hospital de Clínicas (Montevideo, Uruguay) were considered for recruitment in the study. Exclusion criteria were: (a) previous H. pylori treatment; (b) previous gastric surgery; (c) severe systemic illness such as liver cirrhosis or kidney failure; (d) allergy to any of the antibiotics used; (e) pregnancy; (f) age lower than 18 years.
All patients were included consecutively. The most frequent reason for exclusion was self-reported amoxicillin allergy. Written informed consent was obtained from all patients, the ethic committee and the regulatory authority approved the study protocol. Given the high prevalence of H. pylori in Uruguay, in patients with dyspepsia or ulcer the positive predictive value of a single test for Hp infection before treatment is extremely high. A patient was considered to carry H. pylori infection at entry when at least one of the following tests was positive: urea breath test and histology.
Treatment consisted of a 14-day therapy combining two antibiotics – metronidazole 500mg t.i.d. and amoxicillin 1g t.i.d. – plus strong acid inhibition with esomeprazole 40mg b.i.d. Patients were encouraged not to drink alcohol during treatment in order to avoid the possible side effects of the interaction with metronidazole. Eradication was mainly evaluated with a urea breath test except for a few patients with gastric ulcer, who underwent a second endoscopy to rule out gastric cancer. In this case, eradication was assessed by histology. The diagnostic test was performed at least 8 weeks after completion of treatment. Although patients were allowed to use PPI in case of dyspepsia, they were instructed to avoid PPI for at least 2 weeks before the diagnostic test. Therapy compliance and adverse events were assessed by personal interview after the end of antibiotic treatment. We considered adherence as appropriate when the patient reported taking more than 80% of the tablets.
Statistical analysisThe overall eradication rates and their 95% confidence intervals were obtained by ITT and PP. Quantitative variables were given as mean±S.D. A univariate analysis including age (divided into quartiles), sex and indication for treatment was performed using the Chi-square test or the Mann–Whitney U-test. Calculations were performed using the SPSS 21 software.
An expected ITT cure rate of 90% was assumed. A sample of 40 patients was necessary to obtain an estimation of the efficacy of the treatment with a ±5% error margin and a 95% confidence interval.
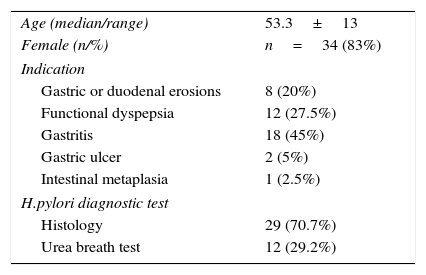
ResultsForty-one patients were included in the study. Demographic and clinical characteristics and the indications for eradication are shown in Table 1. The diagnosis of H. pylori infection previous to treatment was confirmed by histology or urea breathe test. Seventeen per cent of the patients were men and mean age was 53.3±13 years.
Baseline demographic and clinical characteristics of the patients (n=41).
| Age (median/range) | 53.3±13 |
| Female (n/%) | n=34 (83%) |
| Indication | |
| Gastric or duodenal erosions | 8 (20%) |
| Functional dyspepsia | 12 (27.5%) |
| Gastritis | 18 (45%) |
| Gastric ulcer | 2 (5%) |
| Intestinal metaplasia | 1 (2.5%) |
| H.pylori diagnostic test | |
| Histology | 29 (70.7%) |
| Urea breath test | 12 (29.2%) |
Indications for treatment were: functional dyspepsia (27.5%), gastritis (45%), gastric or duodenal erosions (20%), gastric ulcer (5%) and intestinal metaplasia (2.5%). H. pylori eradication was achieved in 33 of the 37 patients who returned for follow-up. Eradication rates were 80.5% (95% CI: 68.4–92.6) by ITT analysis and 89.2% (79.2–99.2) PP (Fig. 1).
Factors influencing the efficacy of therapy were analyzed; there were no statistical differences regarding indication for treatment, sex or age.
Over 95% of the patients reported complete adherence to treatment. The treatment was well tolerated: no major side effects were reported and no patient dropped out due to adverse events. Twenty-six patients (65.8%) presented minor to moderate side effects (nausea, mild diarrhea and headache). All side effects disappeared shortly after the end of treatment.
DiscussionThe present pilot study shows that the triple therapy with strong acid inhibition, metronidazole and amoxicillin in pharmacologically correct schedules during 14 days may represent a suitable alternative to triple standard therapy for H. pylori empirical first-line treatment. The present treatment has shown a promising PP cure rate of 89.2% slightly higher than that obtained with the same schedule treatment during 10 days in a Spanish trial (82.4% (95% CI: 74.7–88.1) by ITT analysis and 88.2% (95% CI: 81.2–92.8) by PP).21 In Uruguay eradication rates with triple standard therapy was described as low as 62.4%.22 Analyzing eradication rates with one of the late trials published in Latin America the efficacy was similar to that obtained with the 14 day triple standard therapy in Greenbergs’trial.9 In the ITT analyses, the probability of eradication with standard therapy was 82.2%, 73.6% with concomitant therapy (only 5 days) and 76.5% with sequential therapy.
Furthermore, cure rates with our triple therapy were similar to those obtained by Molina-Infante et al. in Spain with concomitant quadruple therapy combining a PPI and amoxicillin, metronidazole and clarithromycin for 10 days: 86.4% (95% CI: 76–96) by ITT and 87.6% (CI95% 79–97) by PP.23 Nevertheless, when concomitant therapy was extended to 14 days eradication rates show an ITT and PP cure rates similar or higher than 90%.24,25 Classical quadruple therapy (PPI plus bismuth subcitrate potassium, metronidazole and tetracycline) recorded in a recent randomized European multicenter trial, obtained a 93% by PP, 80% ITT cure rates.11 So the triple therapy assessed here seems as effective as 10 days concomitant quadruple therapy and classical quadruple therapy but less effective than 14 days concomitant quadruple therapy.
The combination of a PPI, amoxicillin and metronidazole has not been widely used because initial studies with PAM administered twice a day for 7 days and at low doses found it to be less effective than clarithromycin-containing triple therapy.26,27 Moreover, resistance to metronidazole in Latin America is even higher.28 For this reason, metronidazole has usually been excluded from first line empirical therapy plans.
This combination has been studies in a recent meta-analysis (with more than 8600 patients).29 Eradication rates were 76% (95% CI: 73–78) per ITT and 80% (95% CI: 78–82) PP. In this meta-analysis 238 patients that received the higher number of antibiotic doses, 14-day treatment and use of high metronidazole doses obtained the higher cure rates 89% (95% CI: 84–94) per ITT and 90% (95% CI: 87–94). Maybe, the benefit of this treatment schedule is higher in settings with lower metronidazole resistance (36% in Uruguay).
In our trial we did not find differences depending on the indication of eradication (ulcer or dyspepsia), ulcer location, sex or age despite the number of patients included was low. Side effects were mild but unusually frequent: twenty-six patients (65.8%) presented with minor to moderate side effects (diarrhea, nausea or headache). This high rate of adverse effects could be due to the treatment lengthening. Using the same therapeutic scheme during 10 days in a Spanish population only 6.6% of the patients presented minor-to-moderate side effects. Most patients completed the treatment despite these mild side effects and the symptoms resolved rapidly after the drugs were stopped. Adherence was very good and 95% of patients reported having completed the prescribed drugs.
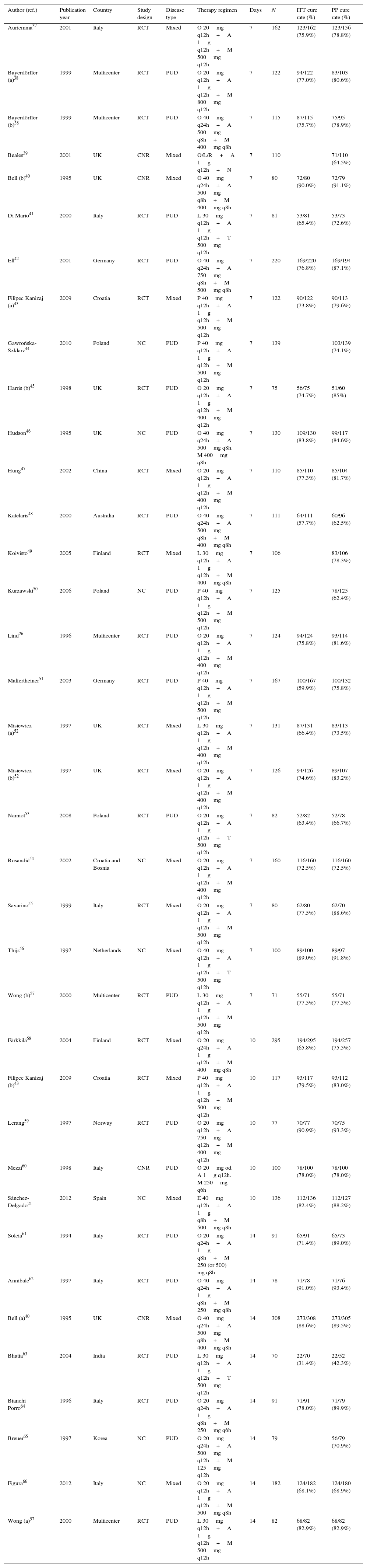
The combination and dose of amoxicillin and metronidazole was strategically selected because it was likely to be useful in areas of high antibiotic resistance. H. pylori resistance to amoxicillin is exceptional.30 Amoxicillin plasma half-life is about an hour and the bactericidal effect is time-dependent. For this reason, amoxicillin was given three times daily to maximize the time above minimal inhibitory concentration. Metronidazole acts by inducing bactericidal DNA double-strand breakage, resulting in cell death.31 The recommended dosage for the majority of infectious diseases is 7.5mg/kg three times a day with a plasma half-life of the molecule between 7 and 10h. In contrast to amoxicillin, H. pylori resistance to metronidazole is high worldwide: “in vitro” resistance rates range from 25% to 50% depending on the area. In Uruguay is as high as 36%.22 Despite this high rate of “in vitro” resistance, metronidazole was chosen because it is unique among the commonly used antibiotics for H. pylori, in that resistance to this drug can be largely overcome by increasing the dose and duration of therapy and by inhibiting gastric acid. Several studies showed good eradication rates using high doses and three times a day schedules of both amoxicillin and metronidazole (Table 2).
Cure rates with amoxicillin plus metronidazole therapies.
| Author (ref.) | Publication year | Country | Study design | Disease type | Therapy regimen | Days | N | ITT cure rate (%) | PP cure rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Auriemma37 | 2001 | Italy | RCT | Mixed | O 20mg q12h+A 1g q12h+M 500mg q12h | 7 | 162 | 123/162 (75.9%) | 123/156 (78.8%) |
| Bayerdörffer (a)38 | 1999 | Multicenter | RCT | PUD | O 20mg q12h+A 1g q12h+M 800mg q12h | 7 | 122 | 94/122 (77.0%) | 83/103 (80.6%) |
| Bayerdörffer (b)38 | 1999 | Multicenter | RCT | PUD | O 40mg q24h+A 500mg q8h+M 400mg q8h | 7 | 115 | 87/115 (75.7%) | 75/95 (78.9%) |
| Beales39 | 2001 | UK | CNR | Mixed | O/L/R+A 1g q12h+N | 7 | 110 | 71/110 (64.5%) | |
| Bell (b)40 | 1995 | UK | CNR | Mixed | O 40mg q24h+A 500mg q8h+M 400mg q8h | 7 | 80 | 72/80 (90.0%) | 72/79 (91.1%) |
| Di Mario41 | 2000 | Italy | RCT | PUD | L 30mg q12h+A 1g q12h+T 500mg q12h | 7 | 81 | 53/81 (65.4%) | 53/73 (72.6%) |
| Ell42 | 2001 | Germany | RCT | PUD | O 40mg q24h+A 750mg q8h+M 500mg q8h | 7 | 220 | 169/220 (76.8%) | 169/194 (87.1%) |
| Filipec Kanizaj (a)43 | 2009 | Croatia | RCT | Mixed | P 40mg q12h+A 1g q12h+M 500mg q12h | 7 | 122 | 90/122 (73.8%) | 90/113 (79.6%) |
| Gawrońska-Szklarz44 | 2010 | Poland | NC | PUD | P 40mg q12h+A 1g q12h+M 500mg q12h | 7 | 139 | 103/139 (74.1%) | |
| Harris (b)45 | 1998 | UK | RCT | PUD | O 20mg q12h+A 1g q12h+M 400mg q12h | 7 | 75 | 56/75 (74.7%) | 51/60 (85%) |
| Hudson46 | 1995 | UK | NC | PUD | O 40mg q24h+A 500mg q8h. M 400mg q8h | 7 | 130 | 109/130 (83.8%) | 99/117 (84.6%) |
| Hung47 | 2002 | China | RCT | Mixed | O 20mg q12h+A 1g q12h+M 400mg q12h | 7 | 110 | 85/110 (77.3%) | 85/104 (81.7%) |
| Katelaris48 | 2000 | Australia | RCT | PUD | O 40mg q24h+A 500mg q8h+M 400mg q8h | 7 | 111 | 64/111 (57.7%) | 60/96 (62.5%) |
| Koivisto49 | 2005 | Finland | RCT | Mixed | L 30mg q12h+A 1g q12h+M 400mg q8h | 7 | 106 | 83/106 (78.3%) | |
| Kurzawski50 | 2006 | Poland | NC | PUD | P 40mg q12h+A 1g q12h+M 500mg q12h | 7 | 125 | 78/125 (62.4%) | |
| Lind26 | 1996 | Multicenter | RCT | PUD | O 20mg q12h+A 1g q12h+M 400mg q12h | 7 | 124 | 94/124 (75.8%) | 93/114 (81.6%) |
| Malfertheiner51 | 2003 | Germany | RCT | PUD | P 40mg q12h+A 1g q12h+M 500mg q12h | 7 | 167 | 100/167 (59.9%) | 100/132 (75.8%) |
| Misiewicz (a)52 | 1997 | UK | RCT | Mixed | L 30mg q12h+A 1g q12h+M 400mg q12h | 7 | 131 | 87/131 (66.4%) | 83/113 (73.5%) |
| Misiewicz (b)52 | 1997 | UK | RCT | Mixed | O 20mg q12h+A 1g q12h+M 400mg q12h | 7 | 126 | 94/126 (74.6%) | 89/107 (83.2%) |
| Namiot53 | 2008 | Poland | RCT | PUD | O 20mg q12h+A 1g q12h+T 500mg q12h | 7 | 82 | 52/82 (63.4%) | 52/78 (66.7%) |
| Rosandić54 | 2002 | Croatia and Bosnia | NC | Mixed | O 20mg q12h+A 1g q12h+M 400mg q12h | 7 | 160 | 116/160 (72.5%) | 116/160 (72.5%) |
| Savarino55 | 1999 | Italy | RCT | Mixed | O 20mg q12h+A 1g q12h+M 500mg q12h | 7 | 80 | 62/80 (77.5%) | 62/70 (88.6%) |
| Thijs56 | 1997 | Netherlands | NC | Mixed | O 40mg q12h+A 1g q12h+T 500mg q12h | 7 | 100 | 89/100 (89.0%) | 89/97 (91.8%) |
| Wong (b)57 | 2000 | Multicenter | RCT | PUD | L 30mg q12h+A 1g q12h+M 500mg q12h | 7 | 71 | 55/71 (77.5%) | 55/71 (77.5%) |
| Färkkilä58 | 2004 | Finland | RCT | Mixed | O 20mg q24h+A 1g q12h+M 400mg q8h | 10 | 295 | 194/295 (65.8%) | 194/257 (75.5%) |
| Filipec Kanizaj (b)43 | 2009 | Croatia | RCT | Mixed | P 40mg q12h+A 1g q12h+M 500mg q12h | 10 | 117 | 93/117 (79.5%) | 93/112 (83.0%) |
| Lerang59 | 1997 | Norway | RCT | PUD | O 20mg q12h+A 750mg q12h+M 400mg q12h | 10 | 77 | 70/77 (90.9%) | 70/75 (93.3%) |
| Mezzi60 | 1998 | Italy | CNR | PUD | O 20mg od. A 1g q12h. M 250mg q6h | 10 | 100 | 78/100 (78.0%) | 78/100 (78.0%) |
| Sánchez-Delgado21 | 2012 | Spain | NC | Mixed | E 40mg q12h+A 1g q8h+M 500mg q8h | 10 | 136 | 112/136 (82.4%) | 112/127 (88.2%) |
| Solcia61 | 1994 | Italy | RCT | PUD | O 20mg q24h+A 1g q8h+M 250 (or 500) mg q8h | 14 | 91 | 65/91 (71.4%) | 65/73 (89.0%) |
| Annibale62 | 1997 | Italy | RCT | PUD | O 40mg q24h+A 1g q8h+M 250mg q8h | 14 | 78 | 71/78 (91.0%) | 71/76 (93.4%) |
| Bell (a)40 | 1995 | UK | CNR | Mixed | O 40mg q24h+A 500mg q8h+M 400mg q8h | 14 | 308 | 273/308 (88.6%) | 273/305 (89.5%) |
| Bhatia63 | 2004 | India | RCT | PUD | L 30mg q12h+A 1g q12h+T 500mg q12h | 14 | 70 | 22/70 (31.4%) | 22/52 (42.3%) |
| Bianchi Porro64 | 1996 | Italy | RCT | PUD | O 20mg q24h+A 1g q8h+M 250mg q6h | 14 | 91 | 71/91 (78.0%) | 71/79 (89.9%) |
| Breuer65 | 1997 | Korea | NC | PUD | O 20mg q24h+A 500mg q12h+M 125mg q12h | 14 | 79 | 56/79 (70.9%) | |
| Figura66 | 2012 | Italy | NC | Mixed | O 20mg q12h+A 1g q12h+M 500mg q8h | 14 | 182 | 124/182 (68.1%) | 124/180 (68.9%) |
| Wong (a)57 | 2000 | Multicenter | RCT | PUD | L 30mg q12h+A 1g q12h+M 500mg q12h | 14 | 82 | 68/82 (82.9%) | 68/82 (82.9%) |
We have only included studies with number of patients greater than 70. NC: not controlled; RCT: randomized controlled trial; CNR: controlled non-randomized; PUD: peptic ulcer disease; NUD: non-ulcer disease; P: pantoprazole; A: amoxicillin; M: metronidazole; O: omeprazole; N: nitroimidazole; Or: ornidazole; T: tinidazole; L: lansoprazole; E: esomeprazole; R: rabeprazole; PPI: proton pump inhibitors; sd: standard dose.
In addition, increasing the intragastric pH to 6 or more allows bacteria to enter the growth phase, thus becoming more sensitive to antibiotics which are effective only against replicating bacteria such as amoxicillin.32 Suppressing acid secretion also increases the concentration of antibiotics in the stomach and its anti-H pylori effect. The importance of achieving adequate acid inhibition has been highlighted by recent studies. Sugimoto et al.33 analyzed 24-h gastric pH during eradication therapy with lansoprazole, clarithromycin and amoxicillin twice daily at standard doses. Cure rates were closely related to the degree of acid inhibition. Interestingly, the infection was cured in all patients who attained a pH above 4 for more than 90% of the time, even in the presence of clarithromycin-resistant strains. It has also been shown that response to PPI is strongly determined by the capacity of the individuals to metabolize the drug. Villoria et al.’s meta-analysis of randomized trials compared a standard dose of a PPI with high-dose PPI, both twice a day in triple therapy.20 The results showed that high doses of a PPI were more effective than standard doses for curing H. pylori infection in 7-day triple therapy. Subgroup analyses showed that the maximum increase was observed when the PPI compared were esomeprazole 40mg vs. omeprazole 20mg or pantoprazole 40mg. This may be because the degree of acid inhibition with new generation PPIs such as esomeprazole and rabeprazole is far more intense than that in the remaining PPIs.34
This pilot study was designed to test the hypothesis that cure rates with esomeprazole, metronidazole and amoxicillin are above 90%, before engaging in a randomized trial. Although the cure rates were among the highest observed in our area, PP cure rates were below the desired figure of 90%.
Given the great results of the 14-day, high-dose concomitant therapy in Spain24,25 or the excellent cure rates of the clarithromycin or metronidazole triple therapies adding bismuth35,36 a comparative study with these three schedules is guaranteed.
The study was performed under clinical practice conditions. Antimicrobial resistance evaluation was not available and many patients were diagnosed by a non-invasive test, so pre-treatment antimicrobial resistance data was not obtained. Resistances to metronidazole are known to be very high in the Uruguayan population, as is usual in Latin American countries.
In conclusion, this combination of 14-day triple therapy combining esomeprazole with high-dose amoxicillin and metronidazole three times a day is well tolerated and seems highly effective in eradicating H. pylori in Uruguay.
Author contributionsStudy concept and design, data collection and analysis, writing the manuscript: Cristina Dacoll, Jordi Sánchez-Delgado and Xavier Calvet; acquisition of data: Cristina Dacoll, H. Balter, X. Pazos, M. Di Pace, G. Sandoya; critical revision of the manuscript for important intellectual content: Cristina Dacoll, Jordi Sánchez Delgado, Xavier Calvet and Henry Cohen. Statistical analysis: Xavier Calvet.
Conflict of interestsThe authors declare that they have no conflict of interest.