Inflammatory Bowel Disease (IBD) treatment may increase the risk of infections. Vaccines are part of the comprehensive IBD patient care. The aim of this study was to describe indications and adherence of immunizations in IBD and identify possible associated factors.
MethodsA cross-sectional, analytic study was conducted in patients from an IBD Program of a tertiary center in Chile, between April – June 2019. Patients were asked to answer a vaccine survey and information also was obtained from the National Immunization Registry. Descriptive and association statistic were used (χ2; p < 0.05).
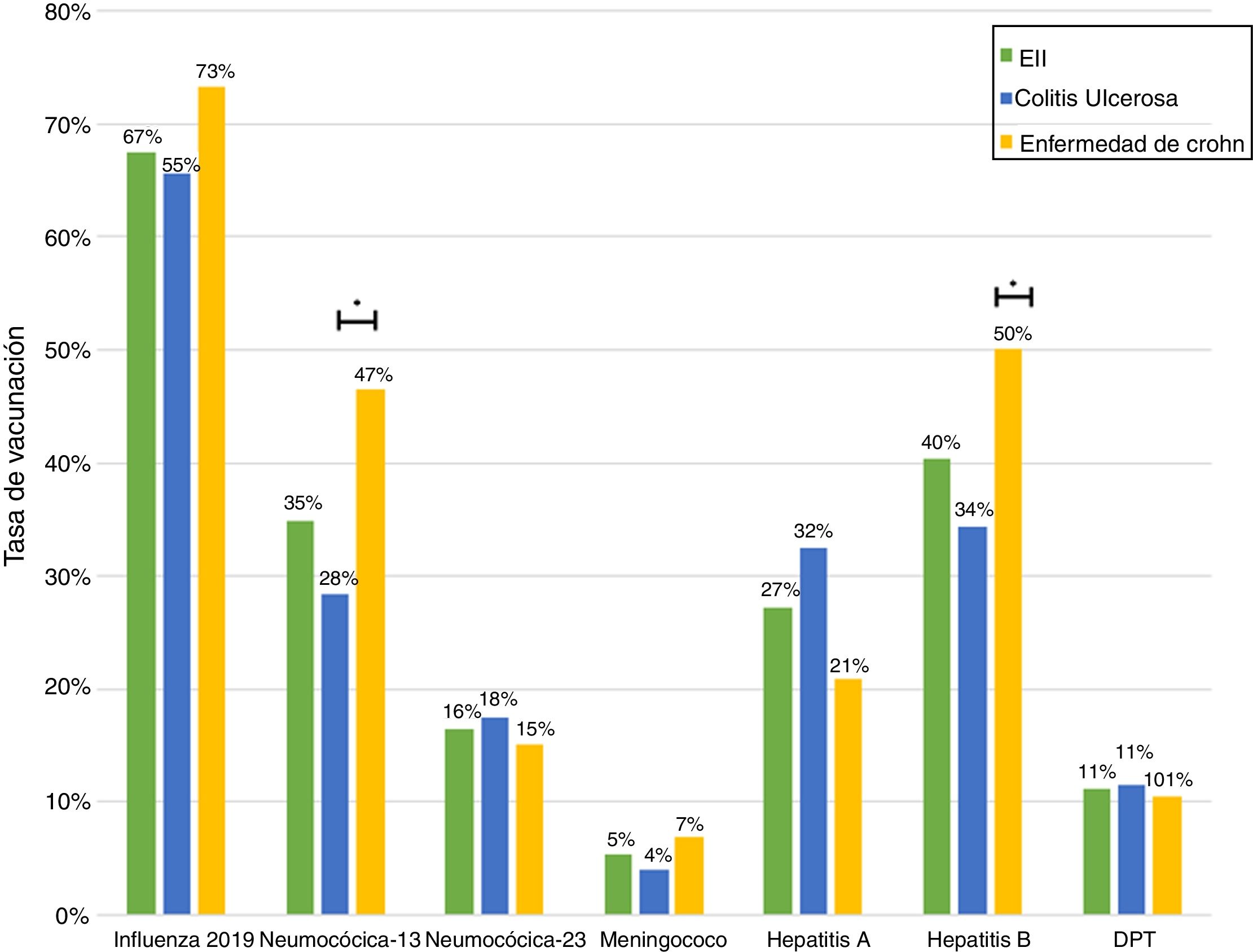
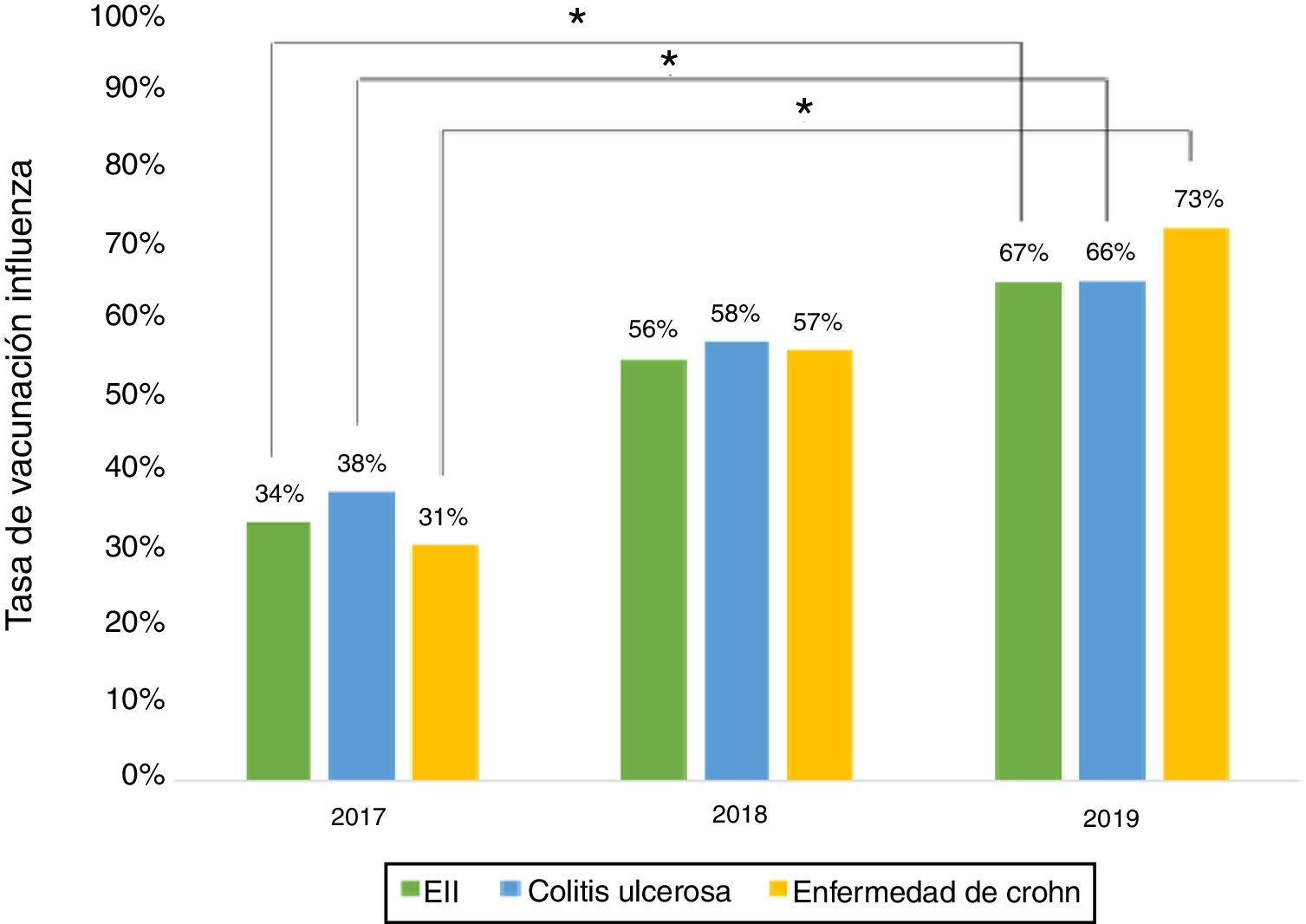
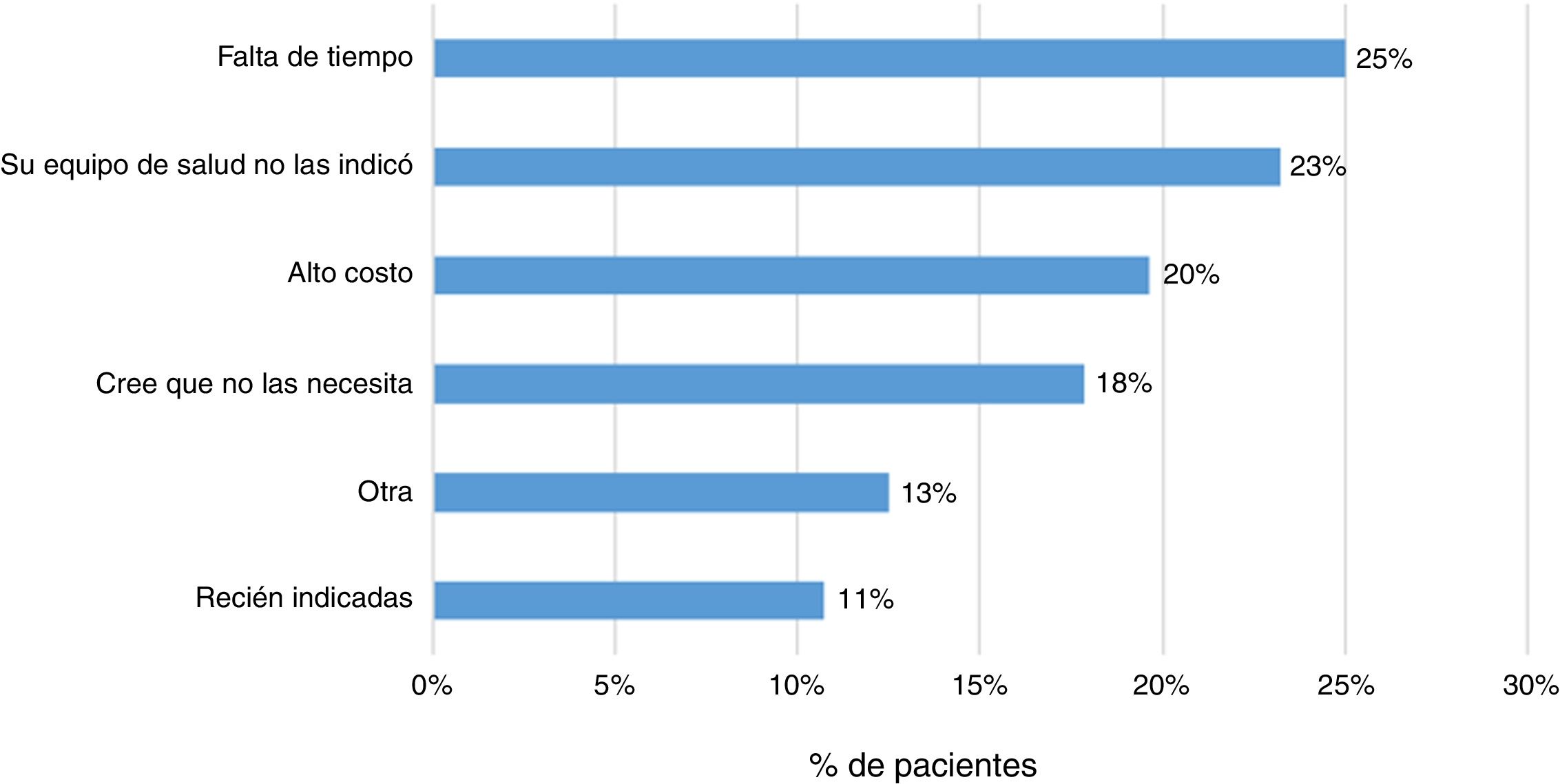
ResultsA total of 243 patients were included (148 ulcerative colitis (UC), 86 Crohn´s disease (CD) and 9 non-classifiable IBD). Only six patients (2%) of IBD patients received a complete immunization schedule. The highest vaccine rates were against influenza (67%), hepatitis B virus (40%), 13-valent pneumococcal (34%) and 23-polysaccharide pneumococcal (16%). The influenza vaccine rate has significantly increased, reaching 67% in 2019. The survey showed that 23% of patients have not been immunized with any vaccine, mainly due to lack of time, lack of medical prescription and high cost.
ConclusionIn this cohort, although vaccination rates are higher than previously reported, adherence to IBD immunization program would be improved, being considered since diagnosis by the multidisciplinary team.
El tratamiento de la Enfermedad Inflamatoria Intestinal (EII) puede aumentar el riesgo de infección. La inmunización es parte del manejo integral de la atención de estos pacientes. El objetivo de este estudio es describir la prescripción y adherencia a la vacunación en pacientes con EII e identificar los posibles factores asociados a ésta.
MétodosEstudio analítico, descriptivo, transversal en pacientes de un Programa de EII de Chile, entre abril-junio 2019. A los pacientes se les solicitó responder un cuestionario acerca de la adherencia a la vacunación. La información de las vacunas se obtuvo del Registro Nacional de Inmunizaciones. Se realizó análisis estadístico descriptivo y de asociación (χ2; p < 0.05).
ResultadosSe incluyeron 243 pacientes con EII (148 Colitis Ulcerosa (CU), 86 enfermedad de Crohn (EC) y 9 EII no-clasificable). Sólo 6 pacientes (2%) recibieron el esquema de inmunización completo. Las vacunas con los mayores porcentajes fueron contra la influenza (67%), virus hepatitis B (40%), neumocócica 13-valente (34%) y neumocócica 23-polisacárida (16%), siendo las dos primeras más frecuentes en EC vs CU (p: <0.05). La administración de la vacuna contra la influenza ha aumentado significativamente, alcanzando un 67% el 2019. La encuesta mostró que el 23% no ha sido inmunizado con ninguna vacuna, principalmente por falta de tiempo, falta de prescripción médica y el alto costo económico.
ConclusionesEn esta cohorte, aunque las tasas de vacunación son más altas que las reportadas previamente, la adherencia al programa de inmunización debe mejorar. siendo consideradas desde el diagnóstico por el equipo multidisciplinario.
Inflammatory bowel disease (IBD), which primarily encompasses Crohn's disease (CD) and ulcerative colitis (UC), are chronic gastrointestinal diseases characterised by alternating periods of relapse and remission. Recently, an increase in the incidence and prevalence of IBD in Latin America and the Caribbean has been demonstrated,1 similar to that seen a few decades ago in developed countries.2 Although Chile lacks data, studies conducted at tertiary hospitals have confirmed increased numbers of cases and hospitalisations in recent years.3,4 In addition, in recent decades, IBD treatment has improved substantially following the incorporation of biologic therapy (TNF inhibitors, anti-integrin therapy and anti-IL-12/IL-23 p40 antibodies) and small molecules.5 While these drugs are effective in managing disease activity and thus achieving clinical and endoscopic remission in a certain percentage of patients, they may also increase risks of infection and reactivation of latent diseases,6,7 given that they affect the humoral and cellular immune system.6
Systematic screening for infections as well as an immunisation schedule for all patients with IBD have been proposed, especially for those who are to receive immunomodulatory therapy (thiopurines/methotrexate), biologics or small molecules.5,6,8–10 The ideal time to immunise is at diagnosis,6,10 regardless of the severity at onset, since disease course and treatment may vary over time. It is important to consider that immunosuppression secondary to drugs may interfere with immunisation efficacy, or contraindicate administration of vaccines made with live agents. Live-attenuated virus vaccines, such as the herpes zoster virus, MMR, varicella and yellow fever vaccines are contraindicated in cases of severe immunosuppression.5,8–10 This group includes patients with IBD who are on treatment with prednisone ≥20 mg/day for more than 2 weeks, immunomodulators, biologic therapies and small molecules.
Although various immunisation schedules have been published,5,6,8–10 vaccine prescription rates in IBD patients remain low.11–15 A study conducted in Canada found that just 61% of IBD patients mentioned having received the influenza vaccine and 10% the pneumococcal vaccine.13 Moreover, a study recently conducted in France, which enrolled 199 patients with IBD, found even lower percentages of immunisation against influenza (34%), and although the percentage of pneumococcal vaccination was higher (38%), it was still low.14 Notable among the factors contributing to this low immunisation rate are the limited knowledge among gastroenterologists about immunisation in IBD, the lack of patient education about vaccines and the role they play, faulty perceptions around vaccine safety in immunocompromised patients, and fear of adverse secondary events.13–18
The objective of this study is to report rates of prescription and adherence to immunisation schedules in IBD patients and identify possible associated factors, comparing this to recommendations in the literature, in order to verify whether vaccination regimens are seeing proper application and compliance in our population.
Patients and methodsAn observational, descriptive, cross-sectional and analytical study conducted in IBD patients who received care in the IBD Programme at Clínica Las Condes [Las Condes Clinic] from April to June 2019.
Patients with a confirmed diagnosis of CD, UC or unclassifiable IBD, with annual follow-up in the IBD Programme at Clínica Las Condes, were invited to fill in a brief questionnaire evaluating the following points: prescription by treating team of an influenza vaccine in the past year, suggestion of other IBD immunisation schedule vaccines, administration of said vaccines and, in the absence thereof, reasons for non-adherence. Information on vaccines administered was drawn from the Registro Nacional de Inmunizaciones [Chilean National Immunisation Registry]. The following vaccines were included in the analysis: influenza, pneumococcal conjugate vaccine (PCV13); pneumococcal polysaccharide vaccine (PPSV23); tetanus, diphtheria, pertussis (DPT); hepatitis A; hepatitis B; human papillomavirus; meningococcal; varicella zoster virus; herpes zoster virus (live); measles, and yellow fever. For the vaccine against influenza, for purposes of comparison, immunisation information received for the years 2017, 2018 and 2019 was assessed.
Demographic variables (age, gender and health coverage system) and clinical variables (diagnosis, Montreal classification, years since disease onset, years of enrolment in the IBD Programme, current treatment and treatment at the time of vaccination) were taken from the registry for purposes of research in patients with IBD at Clínica Las Condes, which was approved by the local Ethics Committee in April 2012.
A full immunisation schedule was defined as administration of the vaccines against hepatitis A, hepatitis B, influenza (within the past year), PCV13, PPSV23, papillomavirus (all patients 11–26 years of age) and herpes zoster virus (all patients ≥50 years of age).
This study was approved by the institution's Ethics Committee and conducted in accordance with the principles of the Declaration of Helsinki.
Statistical analysisThe results of the study were analysed using the R Commander software program. Categorical variables were analysed through absolute frequencies and percentages. Continuous variables were described in terms of measures of central tendency and dispersion depending on data distribution (mean and standard deviation in case of a normal distribution, and interquartile range in case of a non-normal distribution). Continuous variables were compared using the Mann-Whitney U test or Student's t test depending on the distribution. Percentage relative frequency was used for categorical variables, and the chi-squared test was used for comparative statistical analysis. A p value ≤0.05 was considered statistically significant.
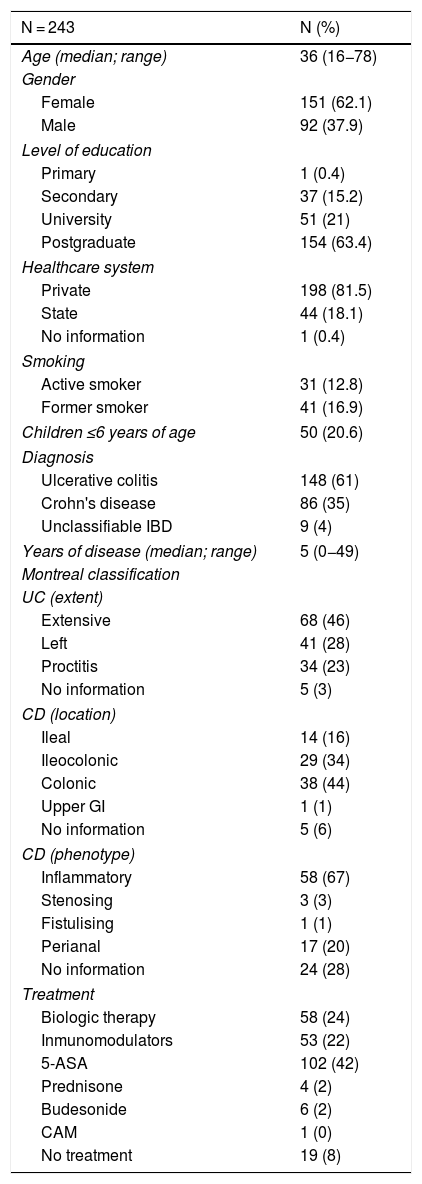
ResultsA total of 243 patients with IBD (corresponding to 53% of all patients assessed in the IBD Programme from April to June 2019) filled in the vaccine survey. There were no exclusions due to incomplete data. The demographic and clinical data for the patients enrolled in the study are shown in Table 1. In total, 148 patients with diagnosed UC, 86 with diagnosed CD and 9 with diagnosed unclassifiable IBD were included in the analysis. The majority were women (68%), with a median age of 36 years (range 18−78), and a median disease duration of 5 years (range 0−49). Most patients had a private health system (82%) and had completed university studies (83%). Among those surveyed, 22% of patients were on immunomodulatory therapy (thiopurines/methotrexate) and 24% were on biologic therapy (43% of patients with CD and 12% with UC). Some 64% of all patients with biologic therapy were on combination treatment with a thiopurine or methotrexate.
Demographic and clinical characteristics of patients with inflammatory bowel disease enrolled in the study.
| N = 243 | N (%) |
|---|---|
| Age (median; range) | 36 (16−78) |
| Gender | |
| Female | 151 (62.1) |
| Male | 92 (37.9) |
| Level of education | |
| Primary | 1 (0.4) |
| Secondary | 37 (15.2) |
| University | 51 (21) |
| Postgraduate | 154 (63.4) |
| Healthcare system | |
| Private | 198 (81.5) |
| State | 44 (18.1) |
| No information | 1 (0.4) |
| Smoking | |
| Active smoker | 31 (12.8) |
| Former smoker | 41 (16.9) |
| Children ≤6 years of age | 50 (20.6) |
| Diagnosis | |
| Ulcerative colitis | 148 (61) |
| Crohn's disease | 86 (35) |
| Unclassifiable IBD | 9 (4) |
| Years of disease (median; range) | 5 (0−49) |
| Montreal classification | |
| UC (extent) | |
| Extensive | 68 (46) |
| Left | 41 (28) |
| Proctitis | 34 (23) |
| No information | 5 (3) |
| CD (location) | |
| Ileal | 14 (16) |
| Ileocolonic | 29 (34) |
| Colonic | 38 (44) |
| Upper GI | 1 (1) |
| No information | 5 (6) |
| CD (phenotype) | |
| Inflammatory | 58 (67) |
| Stenosing | 3 (3) |
| Fistulising | 1 (1) |
| Perianal | 17 (20) |
| No information | 24 (28) |
| Treatment | |
| Biologic therapy | 58 (24) |
| Inmunomodulators | 53 (22) |
| 5-ASA | 102 (42) |
| Prednisone | 4 (2) |
| Budesonide | 6 (2) |
| CAM | 1 (0) |
| No treatment | 19 (8) |
5-ASA: 5-aminosalicylic acid; UC: ulcerative colitis; CD: Crohn's disease; IBD: inflammatory bowel disease; GI: gastrointestinal; CAM: complementary and alternative medicine.
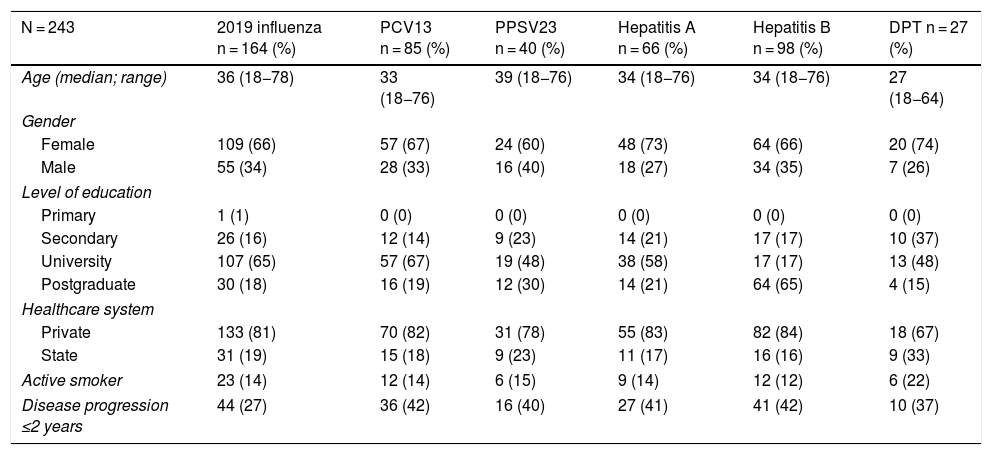
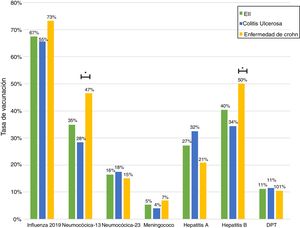
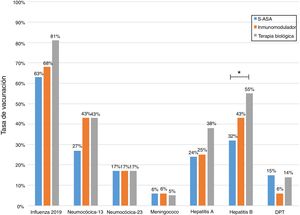
Just 6 patients (2%) received the full immunisation schedule: 3 of them were on combination treatment with biologic therapy and azathioprine, and the other 3 were on treatment with 5-aminosalicylic acid (5-ASA) alone. The vaccines with the highest percentages of administration were the hepatitis B virus vaccine in 98 patients (40%), more often in patients with CD versus UC (50% versus 34%, p = 0.019); PCV13 in 83 patients (34%), more often in CD versus UC (47% versus 28%, p = 0.005); and PPSV23 in 39 patients (16%), with no significant differences between CD and UC (15% versus 18%, p = 0.627). Just 18 patients (7.4%) had received both pneumococcal vaccines. The papillomavirus vaccine was administered in 10 patients, and the herpes zoster vaccine was administered in 7 patients. The percentage of administration of the different vaccines and the demographic and clinical characteristics by type of vaccine received are shown in Fig. 1 and Table 2, respectively.
Demographic and clinical characteristics by type of vaccine received by IBD patients.
| N = 243 | 2019 influenza n = 164 (%) | PCV13 n = 85 (%) | PPSV23 n = 40 (%) | Hepatitis A n = 66 (%) | Hepatitis B n = 98 (%) | DPT n = 27 (%) |
|---|---|---|---|---|---|---|
| Age (median; range) | 36 (18−78) | 33 (18−76) | 39 (18−76) | 34 (18−76) | 34 (18−76) | 27 (18−64) |
| Gender | ||||||
| Female | 109 (66) | 57 (67) | 24 (60) | 48 (73) | 64 (66) | 20 (74) |
| Male | 55 (34) | 28 (33) | 16 (40) | 18 (27) | 34 (35) | 7 (26) |
| Level of education | ||||||
| Primary | 1 (1) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Secondary | 26 (16) | 12 (14) | 9 (23) | 14 (21) | 17 (17) | 10 (37) |
| University | 107 (65) | 57 (67) | 19 (48) | 38 (58) | 17 (17) | 13 (48) |
| Postgraduate | 30 (18) | 16 (19) | 12 (30) | 14 (21) | 64 (65) | 4 (15) |
| Healthcare system | ||||||
| Private | 133 (81) | 70 (82) | 31 (78) | 55 (83) | 82 (84) | 18 (67) |
| State | 31 (19) | 15 (18) | 9 (23) | 11 (17) | 16 (16) | 9 (33) |
| Active smoker | 23 (14) | 12 (14) | 6 (15) | 9 (14) | 12 (12) | 6 (22) |
| Disease progression ≤2 years | 44 (27) | 36 (42) | 16 (40) | 27 (41) | 41 (42) | 10 (37) |
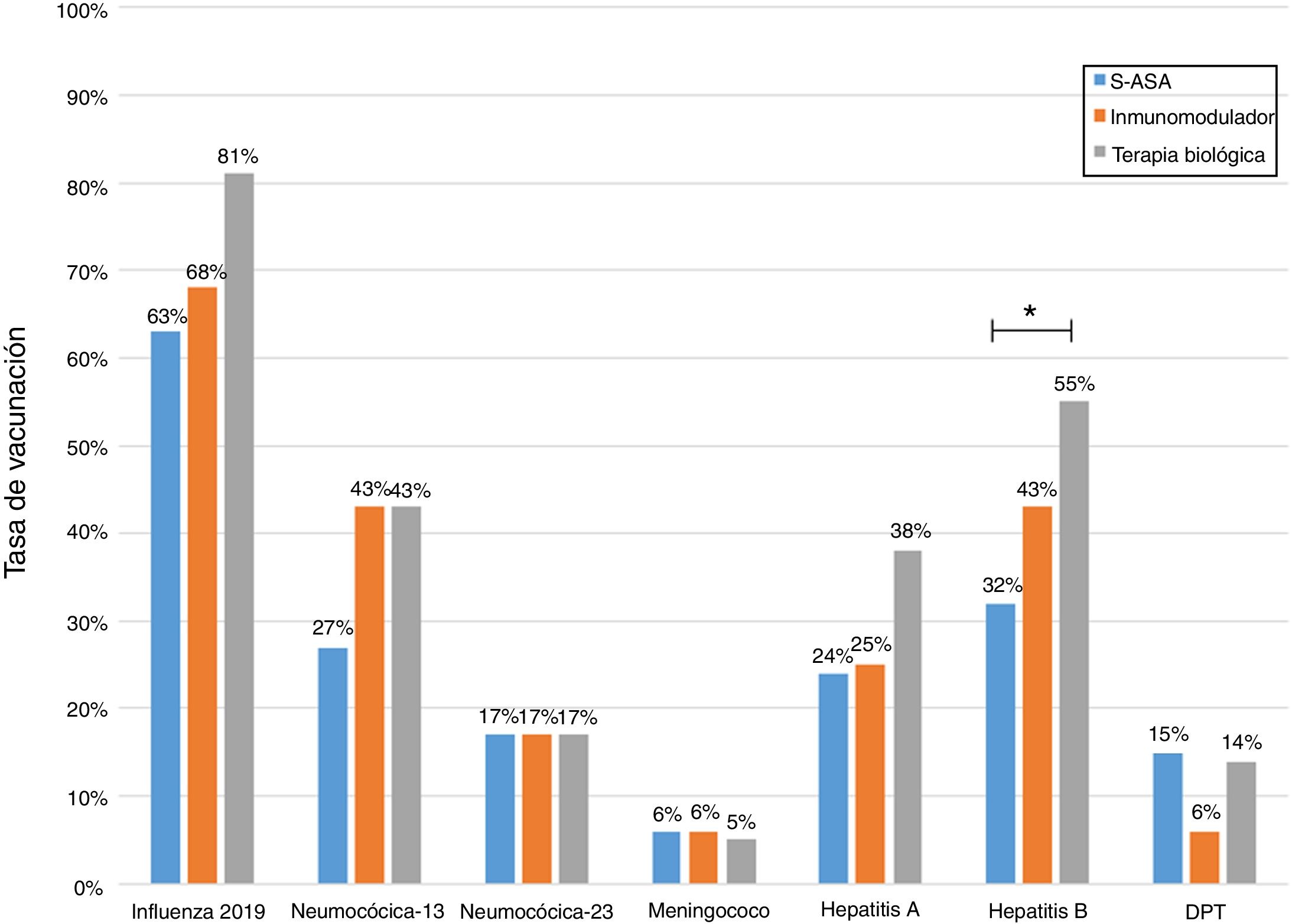
Analysis of immunisation rates by drug groups revealed that the percentage of patients vaccinated against hepatitis B was significantly higher in the group on biologic therapy. For PCV13, all that was observed was a trend towards administration at higher rates in patients on treatment with immunosuppressants or biologics (Fig. 2).
A review of the Registro Nacional de Inmunizaciones indicated that 40 patients received a live-attenuated virus vaccine, and 3 of them even received more than one vaccine. The immunisations received were as follows: 17 against yellow fever, 17 against measles, 7 against herpes zoster and 2 against varicella zoster. Seven of them (18%) were on immunosuppressant treatment at the time of vaccine administration (1 patient was on steroids, 4 were on thiopurines/methotrexate and 2 were on combination treatment with TNF inhibitors and immunomodulators) and none of them received more than one live-attenuated virus vaccine.
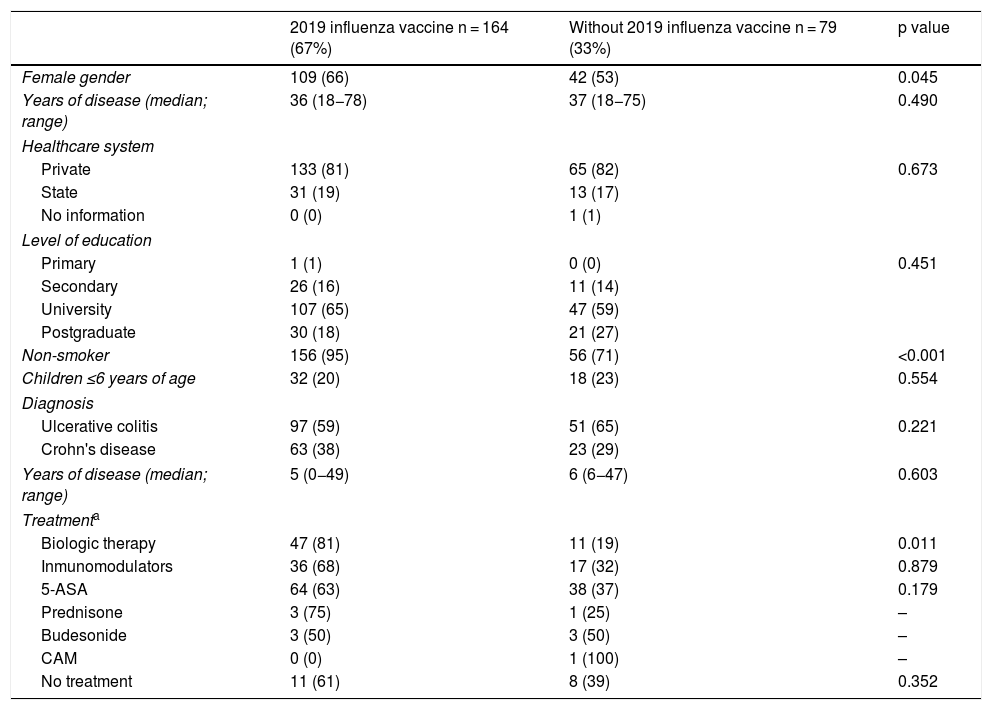
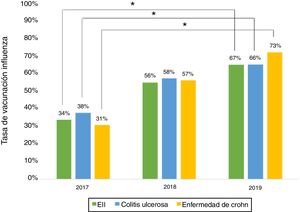
Influenza vaccineRegarding influenza vaccine administration in 2019, patient demographic data is shown in Table 3. The percentage of vaccination was higher in women than in men (66% versus 53%, p = 0.045), in non-smoking versus smoking patients (95% versus 73%, p ≤ 0.001) and patients on biologic therapy versus not (81% versus 19%, p = 0.011). Evaluation of immunisation against influenza carried out in 2017, 2018 and 2019 revealed that the percentage of vaccinated patients had increased significantly in both CD and UC (Fig. 3).
Univariate analysis of demographic and clinical characteristics that could affect influenza vaccination rates in IBD patients.
| 2019 influenza vaccine n = 164 (67%) | Without 2019 influenza vaccine n = 79 (33%) | p value | |
|---|---|---|---|
| Female gender | 109 (66) | 42 (53) | 0.045 |
| Years of disease (median; range) | 36 (18−78) | 37 (18−75) | 0.490 |
| Healthcare system | |||
| Private | 133 (81) | 65 (82) | 0.673 |
| State | 31 (19) | 13 (17) | |
| No information | 0 (0) | 1 (1) | |
| Level of education | |||
| Primary | 1 (1) | 0 (0) | 0.451 |
| Secondary | 26 (16) | 11 (14) | |
| University | 107 (65) | 47 (59) | |
| Postgraduate | 30 (18) | 21 (27) | |
| Non-smoker | 156 (95) | 56 (71) | <0.001 |
| Children ≤6 years of age | 32 (20) | 18 (23) | 0.554 |
| Diagnosis | |||
| Ulcerative colitis | 97 (59) | 51 (65) | 0.221 |
| Crohn's disease | 63 (38) | 23 (29) | |
| Years of disease (median; range) | 5 (0−49) | 6 (6−47) | 0.603 |
| Treatmenta | |||
| Biologic therapy | 47 (81) | 11 (19) | 0.011 |
| Inmunomodulators | 36 (68) | 17 (32) | 0.879 |
| 5-ASA | 64 (63) | 38 (37) | 0.179 |
| Prednisone | 3 (75) | 1 (25) | – |
| Budesonide | 3 (50) | 3 (50) | – |
| CAM | 0 (0) | 1 (100) | – |
| No treatment | 11 (61) | 8 (39) | 0.352 |
5-ASA: 5-aminosalicylic acid; IBD: inflammatory bowel disease; CAM: complementary and alternative medicine.
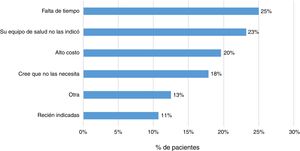
Some 83% (202 patients) indicated that their treating physician had prescribed them the vaccine against influenza, and 79% indicated the same regarding some other vaccine. In addition, 23% (57 patients) responded that they had not received any sort of immunisation. The main reasons indicated by patients were lack of time (25%), lack of prescription (23%) and high cost (20%) (Fig. 4).
DiscussionVarious treatments, be they steroids, immunomodulators, biologic therapies and/or small molecules, carry risks of developing infection that vary by drug.19 These risks can be prevented with a suitable immunisation schedule.5,6,8–10 Several studies have pointed to a higher risk of fulminant infections and mortality due to hepatitis B, varicella and pneumococcal infection in immunosuppressed IBD patients.20–23 Routine vaccination of IBD patients with is recommended, although, as an exception, vaccines that contain live attenuated viruses should be avoided in immunocompromised patients.5,6,8–10 It has been suggested that immunisations be assessed at diagnosis in order to indicate all pending vaccines.9,10 It should be emphasised that a suitable immunisation schedule, and adherence thereto, is considered an indicator of care quality in patients with IBD.24,25
Our results show a significant deficiency in immunisation schedules in IBD patients, with percentages of vaccination being as high as 66% for the influenza vaccine and as low as 5% for the meningococcal vaccine, the latter in a risk population.5,6,8–10 These findings are similar to those reported in other studies.11,26 It has been put forward that all patients with IBD should receive both vaccines. Ideally, they should receive PCV13 first and PPSV23 two months later, with a booster of the latter after 5 years.9,10 In our study, just 7.4% received both pneumococcal vaccines. In addition, even lower rates of vaccination against influenza have been shown in other studies, such as a study by Loubet et al., in which just 34% of patients received this vaccine.14 Finally, other studies have found that 45% of patients with IBD had received the full vaccination schedule.13 In our study, just 6 patients (2%) received an immunisation schedule with all recommended vaccines.
Several studies have shown that the extent to which patients are informed about their disease is lacking,27,28 and in one study 58% of patients reported having inadequate information on the need to get vaccinated against influenza and pneumococcal infection.14 In addition, there is a lack of knowledge of vaccines on the part of treating teams, and there is uncertainty among patients around indications for vaccines.16–18 Undoubtedly, these two variables must be considered, since they are predictors of suitable adherence to immunisation schedules and other preventive interventions in IBD patients.26,29 In a study by Malhi et al.,13 just 54% of patients on biologic therapy remembered having discussed the need for vaccination before starting this treatment strategy, and just 60% were instructed to get vaccinated against influenza. Our study found that patients on biologic therapy had a higher percentage of vaccination against influenza versus patients on other drugs. This could be linked to the larger volume of care provided and the resulting higher level of exposure by the programme team, which could be associated with a better level of education30 and the fact that getting this vaccine is a requirement for access to Law 20.850 ("Ricarte Soto Law"), a Chilean state benefit that allows IBD patients access to biologic therapy with TNF inhibitors.
Concerning percentages of vaccination by diagnosis, our results showed that administration of the vaccine against hepatitis B virus and administration of PCV13 were more common in patients with CD versus UC. This was consistent with that reported in other studies.15 The higher percentage of administration of these vaccines in CD might be explained by the larger number of patients with this disease being treated with biologic therapy and therefore requiring more care from the treating team. It is important to consider that, in Chile, Law 20.850 came into force for patients with CD in January 2017 and for patients with UC in July 2019. This might have had an impact on the higher percentage of patients with CD treated with these drugs.
The percentage of vaccination against influenza increased significantly from 2017 to 2019, hitting 66% in 2019. This increase might be tied to patient education efforts and recent nationwide influenza vaccination campaigns in Chile. Similar situations have been cited in other studies.15
It has been found that there are myths about vaccine safety and that there is a need for more education of patients with IBD.13 In our study, 40 patients received a live-attenuated virus vaccine and 7 of them (18%) were on immunomodulatory treatment. In Malhi et al.'s study, 70% of patients on immunomodulatory therapy were never told that this vaccine was contraindicated.13 This was despite the fact that guidelines and reviews on immunisation in IBD patients clearly state that live-attenuated virus vaccines are contraindicated in patients on treatment with this type of drug.5,6,8–15
Finally, it was found that 23% of patients had not been immunised with any vaccine. The main reasons reported by patients were lack of time, lack of prescription and high cost. It has been indicated that uncertainty around vaccine indication, effectiveness and secondary adverse events are factors that may lead to poor immunisation schedule adherence.13,14
Our study has certain strengths. As it is a cross-sectional study and shares the experience of a tertiary hospital with an established IBD programme, it offers a view of daily clinical practice with a variety of stages of progression of both CD and UC and with different treatment strategies. This local experience was assessed in 53% of patients being followed up in our IBD programme, which enables comparison of our results to other studies. Finally, to avoid memory bias, a limitation of all retrospective studies that include surveys, the Registro Nacional de Inmunización was reviewed to gather information about each patient's vaccination. On the other hand, this study also has some limitations. it is a study conducted at a tertiary hospital, meaning that it probably was biased towards patients with more serious disease and a higher percentage of immunomodulatory and/or biologic therapy. Therefore, perceptions around immunisation may not reflect what happens in the general IBD population cared for at other hospitals in Chile.
In conclusion, our study suggests that immunisation programmes in IBD patients are still deficient. As current treatments and future treatment strategies place these patients at risk, education of these patients and of the treating team is essential in order to improve adherence to vaccination programmes. The treating team should play an active role in the comprehensive care of IBD patients.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Quera R, Simian D, Núñez P, Flores L, Figueroa C, Ibáñez P, et al. ¿Están recibiendo los pacientes con enfermedad inflamatoria intestinal una adecuada inmunización? Gastroenterol Hepatol. 2021;44:198–205.