The cytoplasmic rods-rings (RR) pattern is found in hepatitis C (HCV) patients treated with interferon-ribavirin when studied with ANA-IIF. Ribavirin aggregates/induces antigenic changes in IMPDH-2, an enzyme necessary for ribavirin action.
Patients and methodProspective search for anti-RR autoantibodies (HEp-2, INOVA) in patients treated with direct-acting antivirals (DAAs) from October 2015 to June 2017. HCV-negative patients from up to June 2016 acted as controls. Anti-RR was analyzed at baseline and, mainly, during treatment and follow-up. The Chi-square test, Student's t-test and a logistic regression analysis were performed.
ResultsBetween October 2015 and June 2016, 1258 men and 2389 women who were HCV-negative and 137 men and 112 women who were HCV-positive patients were studied. Approximately 22.9% of HCV-negative and 13.2% of HCV-positive were ANA-IIF-positive (p<0.05). Three HCV-negative (0.08%) and 23 (9.2%) HCV-positive patients had anti-RR (p<0.001). A total of 122 patients received DAAs; 30 received DAA+RBV; 46 pre-treated with IFN-RBV received DAA; 31 pre-treated with IFN-RBV received DAA+RBV; 16 received IFNpeg-RBV; and 24 received IFN-RBV-DAA. None of the 122 DAA-treated patients showed anti-RR; anti-RR were identified in 14.8% of those treated with DAA-RBV; in 25.9% of those pre-treated with IFN-RBV receiving DAA; in 22.2% of IFN-RBV-pre-treated patients who received DAA+RBV; in 7.4% of those treated with IFNpeg-RBV and in 29.6% of those treated with IFNpeg-RBV-DAA. The multivariate analysis showed significant associations between anti-RR and “Exposure to IFN” and “Time of exposure to RBV”.
ConclusionsAnti-RR autoantibodies were detected only in patients with current or past treatments with RBV, even in cases in which only DAAs were later administered.
La investigación de ANA-IFI en pacientes con hepatitis C tratados con interferón-ribavirina ha detectado un patrón citoplasmático en bastones y anillos. La ribavirina agregaría e induciría cambios antigénicos en la IMPDH-2, enzima imprescindible para su acción.
Pacientes y métodoInvestigar las tasas de anti-RR (HEp-2, INOVA) en pacientes tratados con antivirales de acción directa (AAD) entre octubre-2015 y junio-2017. Como controles se han utilizado los pacientes VHC-negativo habidos hasta junio-2016. Los anti-RR se analizaron antes del tratamiento y, mayoritariamente, durante y después del mismo. Se han utilizado las pruebas Chi-cuadrado, T de Student y de regresión logística.
ResultadosEntre octubre-2015 y junio-2016 hubo 1.258 varones y 2.389 mujeres VHC-negativo y 137 varones y 112 mujeres VHC-positivo. El 22,9% de los VHC-negativo y el 13,2% de los VHC-positivo fueron ANA-IFI-positivo (p<0,05). Tres pacientes (0,08%) VHC-negativo y 23 (9,2%) VHC-positivo fueron anti-RR+ (p<0,001). Ciento veintidós pacientes recibieron AAD; 30, AAD+RBV; 46 pretratados con IFN-RBV recibieron AAD; 31, pretratados con IFN-RBV, recibieron AAD+RBV; 16 IFNpeg-RBV; 24 IFN-RBV-AAD. Ningún paciente con AAD mostró anti-RR; tuvieron anti-RR el 14,8% de los tratados con AAD+RBV; 25,9% de los pretratados con IFN-RBV que recibieron AAD; 22,2% de los pretratados con IFN-RBV que recibieron AAD+RBV; 7,4% de los tratados con IFNpeg-RBV; el 29,6% de los tratados con IFNpeg-RBV-AAD. El análisis multivariante asoció significativamente la presencia de anti-RR con «Exposición al IFN» y «Tiempo de exposición a ribavirina».
ConclusionesLos autoanticuerpos anti-RR solo se han detectado en pacientes tratados con ribavirina, incluso cuando después se utilizaron solamente AAD.
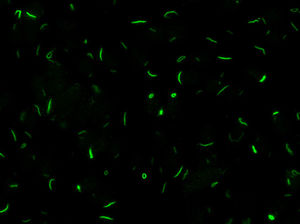
Chronic hepatitis C virus (HCV) infection is a process associated with the appearance of autoimmune complications such as arthritis, glomerulonephritis and mixed crioglobulinemia, hypothyroidism, Sjögren syndrome and type 1 diabetes.1 Another common feature is the production of organ-specific auto-antibodies against thyroid cells, pancreatic islets or parietal gastric cells, and non-organ-specific antibodies such as antinuclear, anti-smooth muscle, anti-microsomal (anti-LK1), anti-mitochondrial and anti-neutrophil cytoplasmic antibodies, which occur in up to 50% of these patients.2 In 2011, Seelig et al.3 and Carcamo et al.4 identified a distinct cytoplasmic pattern of rods and rings (RR), when studying the presence of anti-nuclear antibodies by indirect immunofluorescence (ANA-IIF) on HEp-2 cells, in patients with chronic hepatitis C who had been treated with interferon (IFN) and ribavirin (RBV). This pattern appeared when using the HEp-2 cell substrate of INOVA (San Diego, California) and Euroimmun (Lübeck, Germany),4,5 but not in HEp-2 substrates of a different commercial origin or with “in house” HEp-2 cells. The antigen corresponds to a cytoplasmic aggregation of the inosin-5′-monophosphate-dehydrogenase-2 enzyme (IMPDH-2), essential for the synthesis of guanosine-monophosphate (GMP) and nucleic acids. The treatment of cultures that do not express RR with IMPDH-2 inhibitors, such as mycophenolic acid and RBV, or with inhibitors of cytidin-triphosphate-synthase-1 (CTPS-1), necessary for the synthesis of nucleic acids, such as DON, acivicin or RBV, systematically induce the appearance of this pattern. Ultrastructural studies have shown that RR consist of fibrous cytoplasmic aggregates, without a surrounding membrane, composed of regularly repeated subunits of 10.94±0.82nm; they also confirmed the presence of a single rod (“rod”) in the cell nucleus.6
The rods and ring pattern in HCV infection has been detected almost exclusively in patients treated with IFN and RBV. RBV-monophosphate appears to compete with inosin-5′-monophosphate for IMPDH-2, inhibiting this enzyme, causing its cytoplasmic aggregation and possibly inducing the exposure of antigenic determinants, while IFN appears to act by cooperating with the immune stimulation. Covini et al. found significantly higher rates of anti-RR autoantibodies in non-responders (NR) or relapsers (REL) than in those with sustained virological response (SVR) (33% vs. 11%; p=0.037).7 Novembrino et al. have shown that the anti-RR pattern was higher in REL than in NR-SVR (56% vs. 30%; p=0.082).8 In this study, the rate of autoantibodies increased slowly after exposure to RBV, reaching peak rates of around 50% at the end of the treatment, and declined gradually after treatment cessation. The same behavior of antibody titers have been described by other authors.9 Up to now, there has not been any marker relating the presence of anti-RR with HCV-associated autoimmune diseases, either hepatic or extrahepatic.
In 2015, the new direct-acting antiviral agents (DAA) were incorporated into the treatment of chronic hepatitis C in IFN-free regimes, with or without RBV. With this in mind, we designed the present study to analyze the frequency of anti-RR antibodies in patients with chronic hepatitis C treated with DAA either alone or in combination with RBV.
Material and methodBackgroundIn January 2013 our laboratory changed the technique employed to study the presence of anti-cellular antibodies by ANA-IIF, replacing the ImmunoConcepts, Inc. (Sacramento, California) analytical system to the more standard procedure of INOVA (NOVA-lite, INOVA Diagnostics, San Diego, California). In both cases, a cell line of human laryngeal cancer (HEp-2 cells) was used as a substrate, the main difference lying in the system of fixation of the cells on the slide with acetone. After introducing this change, we identified anti-RR for the first time. The routine study of anti-cellular antibodies in patients with chronic hepatitis C was initiated in January 2015 and the present study began on October 1, 2015.
Study designThis descriptive cohort study was designed to define the associated risk of developing anti-RR using DAA in comparison with other treatments of chronic hepatitis C. As a secondary objective, we aimed to assess the influence of previous treatment with IFN-RBV on the appearance of anti-RR antibodies.
PatientsThe first phase of the investigation lasted from October 1, 2015 to June 30, 2016. In this phase, all patients whose blood samples had arrived at the laboratory of the Parc Hospitalari Martí i Julià, Salt, Girona, for the study of ANA-IIF were assessed. The samples were divided into two groups according to whether or not patients were carriers of the hepatitis C virus. A careful assessment concerning the HCV status was carried out on a patient-to-patient basis. Patients with chronic hepatitis C were from the outpatient practice of the Department of Digestive Diseases, Hospital Doctor Josep Trueta, Girona. The second phase lasted from July 1, 2016 to June 30, 2017, and all new patients diagnosed with chronic hepatitis C in this period were added to the hepatitis C infection group. The chronic hepatitis C group included patients who had recently started treatment, those in whom treatment was underway and those in follow-up. Blood samples were obtained at baseline and during the treatment, being the total number of follow-up samples left at the discretion of the responsible physician. The study closed on June 30, 2017 when new DAA combinations made difficult-to-treat patients unsuitable to receive RBV. In patients with chronic hepatitis C the following data were recorded: date of birth, gender, genotype, IL-28B, ANA-IIF pretreatment, presence of anti-RR antibodies, anti-HCV treatment, type of treatment, previous treatment with IFN-RBV, exposure to IFN, exposure to RBV, exposure to DAA and duration of exposure to IFN (weeks), RBV (weeks) and DAA (weeks).
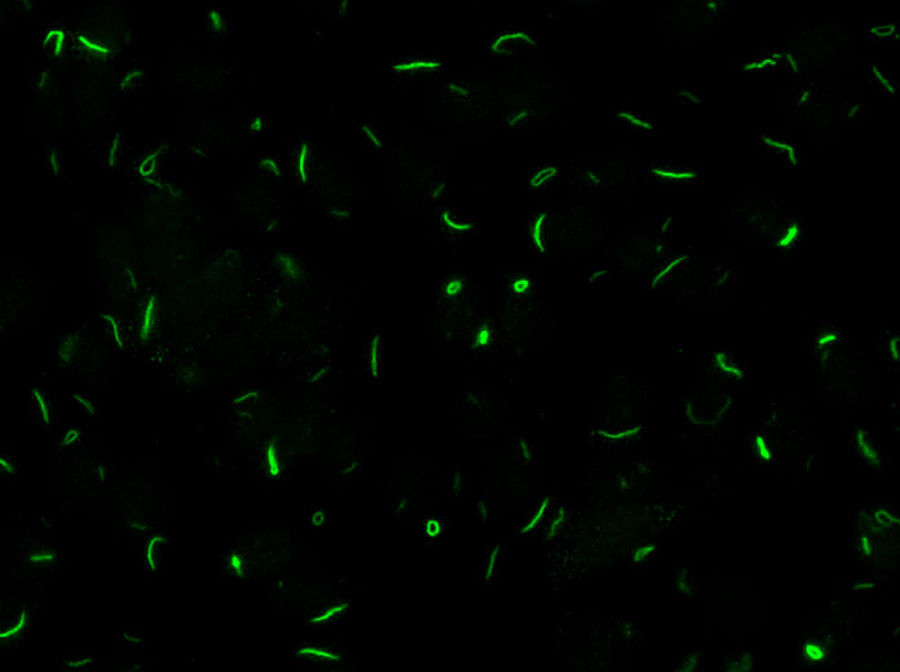
LaboratoryDetection of anti-cellular antibodies was performed by IIF in HEp-2 cells (NOVA-lite, INOVA Diagnostics, San Diego, California) according to the recommended protocol that included a baseline screening dilution of 1/80. Plates were prepared in the QUANTA-Lyser system and read in an automated fashion with the NOVA-View system. The detection of a positive ANA-IIF pattern, in either the cell nucleus or the cytoplasm, was followed by two-fold serial dilutions with an anti-RR ending dilution of 1/1280. The rods and rings pattern corresponded with the AC-23 pattern of the ICAP classification (ICAP: International Consensus on ANA patterns) (Fig. 1).
Statistical analysisQualitative variables were summarized as absolute values and percentages and quantitative variables as means and standard deviations. The Chi-square test, or Fisher's exact test, was used to analyze categorical variables and the Student t test to analyze quantitative variables. Binary logistic regression was performed to determine factors associated with presence of rods & rings. Results were expressed by means of odds ratios and 95% confidence intervals. All tests were 2-tailed and a p-value less than 0.05 was considered statistically significant. For the data analysis, the IBM SPSS Statistics 25 and Stata 13 were used.
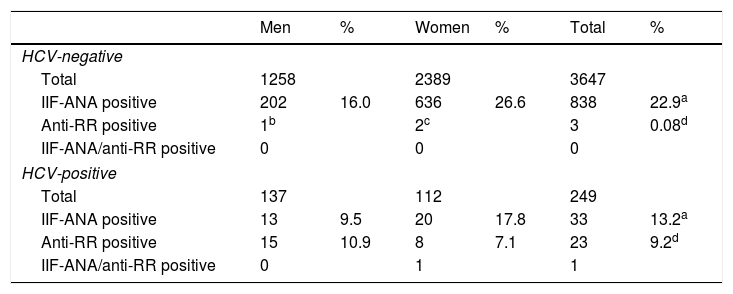
ResultsBetween October 1, 2015 and June 30, 2016, 4679 samples were obtained corresponding to 3647 patients (1258 men, 2389 women) without HCV infection and 708 samples corresponding to 249 patients (137 men, 112 women) with HCV infection. In all, 22.9% of patients without HCV infection (16.0% men, 26.6% women) and 13.2% (9.5% men, 17.8% women) with chronic hepatitis C were ANA-IIF-positive (p<0.05). Only three HCV-negative patients (0.08%) were anti-RR-positive while in the chronic hepatitis C group, 23 (9.2%) had anti-RR antibodies (p<0.001), a pattern that was more frequent in men than in women (10.9% vs.7.1%; p=0.057) (Table 1).
Patients from october-1, 2015 to June-30, 2016.
| Men | % | Women | % | Total | % | |
|---|---|---|---|---|---|---|
| HCV-negative | ||||||
| Total | 1258 | 2389 | 3647 | |||
| IIF-ANA positive | 202 | 16.0 | 636 | 26.6 | 838 | 22.9a |
| Anti-RR positive | 1b | 2c | 3 | 0.08d | ||
| IIF-ANA/anti-RR positive | 0 | 0 | 0 | |||
| HCV-positive | ||||||
| Total | 137 | 112 | 249 | |||
| IIF-ANA positive | 13 | 9.5 | 20 | 17.8 | 33 | 13.2a |
| Anti-RR positive | 15 | 10.9 | 8 | 7.1 | 23 | 9.2d |
| IIF-ANA/anti-RR positive | 0 | 1 | 1 | |||
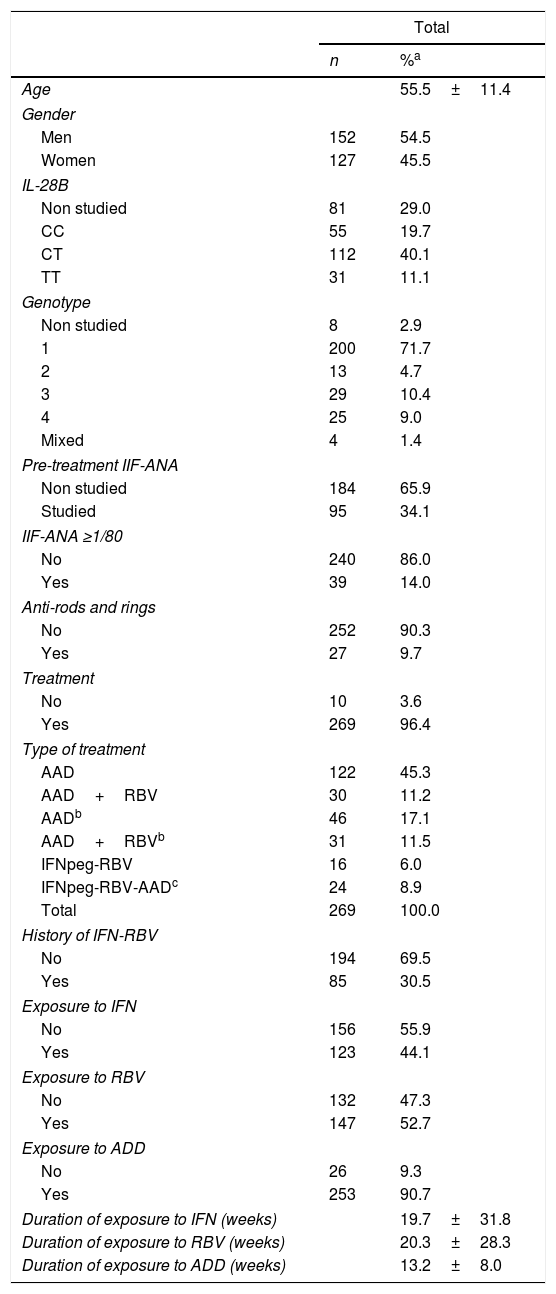
In the second phase of the study (Table 2), between 30 July 2016 and 30 June 2017, 30 new patients (56 blood samples, 15 men, 15 women) with chronic hepatitis C were added to the hepatitis group, making a total of 279 patients. The presence of anti-nuclear antibodies was analyzed prior to treatment in 34% of patients; 14% of those studied for ANA-IIF were positive, either before treatment or at any point in the follow-up. Anti-RR autoantibodies were present in 9.7% of patients; three of them, one in the first phase and two in the second, were positive for both auto-antibodies. After the initial evaluation, 10 patients were not treated and all of them were ANA-IIF and anti-RR negative. Of the 269 patients treated, 122 received only DAA; 30 patients received only DAA-RBV; 46 patients, previously treated with IFN-RBV, received DAA; 31 patients, previously treated with IFN-RBV, received DAA-RBV; 16 patients were treated with IFNpeg-RBV; finally, 24 patients were treated with IFNpeg-RBV plus some DAA. This last group included eight patients with a history of treatment with IFN-RBV. In all, 85 patients (30.5%) included in the study had received prior treatment with IFN-RBV (Table 2).
Characteristics of patients with chronic hepatitis C (Phase I plus Phase II).
| Total | ||
|---|---|---|
| n | %a | |
| Age | 55.5±11.4 | |
| Gender | ||
| Men | 152 | 54.5 |
| Women | 127 | 45.5 |
| IL-28B | ||
| Non studied | 81 | 29.0 |
| CC | 55 | 19.7 |
| CT | 112 | 40.1 |
| TT | 31 | 11.1 |
| Genotype | ||
| Non studied | 8 | 2.9 |
| 1 | 200 | 71.7 |
| 2 | 13 | 4.7 |
| 3 | 29 | 10.4 |
| 4 | 25 | 9.0 |
| Mixed | 4 | 1.4 |
| Pre-treatment IIF-ANA | ||
| Non studied | 184 | 65.9 |
| Studied | 95 | 34.1 |
| IIF-ANA ≥1/80 | ||
| No | 240 | 86.0 |
| Yes | 39 | 14.0 |
| Anti-rods and rings | ||
| No | 252 | 90.3 |
| Yes | 27 | 9.7 |
| Treatment | ||
| No | 10 | 3.6 |
| Yes | 269 | 96.4 |
| Type of treatment | ||
| AAD | 122 | 45.3 |
| AAD+RBV | 30 | 11.2 |
| AADb | 46 | 17.1 |
| AAD+RBVb | 31 | 11.5 |
| IFNpeg-RBV | 16 | 6.0 |
| IFNpeg-RBV-AADc | 24 | 8.9 |
| Total | 269 | 100.0 |
| History of IFN-RBV | ||
| No | 194 | 69.5 |
| Yes | 85 | 30.5 |
| Exposure to IFN | ||
| No | 156 | 55.9 |
| Yes | 123 | 44.1 |
| Exposure to RBV | ||
| No | 132 | 47.3 |
| Yes | 147 | 52.7 |
| Exposure to ADD | ||
| No | 26 | 9.3 |
| Yes | 253 | 90.7 |
| Duration of exposure to IFN (weeks) | 19.7±31.8 | |
| Duration of exposure to RBV (weeks) | 20.3±28.3 | |
| Duration of exposure to ADD (weeks) | 13.2±8.0 | |
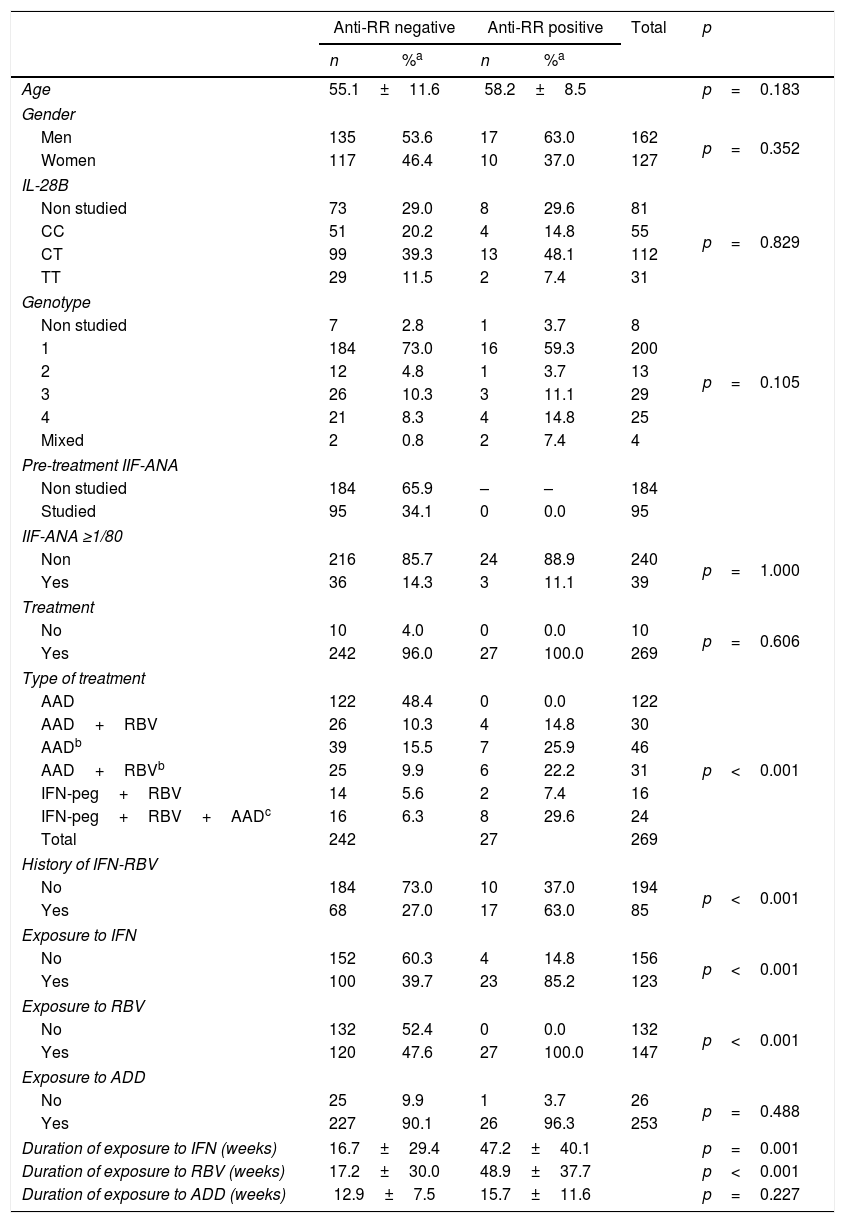
Table 3 shows the distribution of patients in relation to the presence of anti-RR auto-antibodies. Neither the 10 untreated patients nor the 95 patients with ANA-IIF studies prior to the treatment showed the RR pattern. Similarly, none of the 122 patients treated with DAA alone was anti-RR. On the other hand, anti-RR auto-antibodies appeared in 14.8% of DAA-RBV-treated patients, in 25.9% of those who received DAA but had previously been treated with IFN-RBV and in 22.2% of those who received DAA-RBV and had also been treated with IFN-RBV. In addition, 7.4% of those treated with IFNpeg-RBV and 29.6% of those treated with IFN-RBV-DAA were anti-RR-positive.
Distribution of patients with chronic hepatitis C according to their anti-RR status.
| Anti-RR negative | Anti-RR positive | Total | p | |||
|---|---|---|---|---|---|---|
| n | %a | n | %a | |||
| Age | 55.1±11.6 | 58.2±8.5 | p=0.183 | |||
| Gender | ||||||
| Men | 135 | 53.6 | 17 | 63.0 | 162 | p=0.352 |
| Women | 117 | 46.4 | 10 | 37.0 | 127 | |
| IL-28B | ||||||
| Non studied | 73 | 29.0 | 8 | 29.6 | 81 | p=0.829 |
| CC | 51 | 20.2 | 4 | 14.8 | 55 | |
| CT | 99 | 39.3 | 13 | 48.1 | 112 | |
| TT | 29 | 11.5 | 2 | 7.4 | 31 | |
| Genotype | ||||||
| Non studied | 7 | 2.8 | 1 | 3.7 | 8 | p=0.105 |
| 1 | 184 | 73.0 | 16 | 59.3 | 200 | |
| 2 | 12 | 4.8 | 1 | 3.7 | 13 | |
| 3 | 26 | 10.3 | 3 | 11.1 | 29 | |
| 4 | 21 | 8.3 | 4 | 14.8 | 25 | |
| Mixed | 2 | 0.8 | 2 | 7.4 | 4 | |
| Pre-treatment IIF-ANA | ||||||
| Non studied | 184 | 65.9 | – | – | 184 | |
| Studied | 95 | 34.1 | 0 | 0.0 | 95 | |
| IIF-ANA ≥1/80 | ||||||
| Non | 216 | 85.7 | 24 | 88.9 | 240 | p=1.000 |
| Yes | 36 | 14.3 | 3 | 11.1 | 39 | |
| Treatment | ||||||
| No | 10 | 4.0 | 0 | 0.0 | 10 | p=0.606 |
| Yes | 242 | 96.0 | 27 | 100.0 | 269 | |
| Type of treatment | ||||||
| AAD | 122 | 48.4 | 0 | 0.0 | 122 | p<0.001 |
| AAD+RBV | 26 | 10.3 | 4 | 14.8 | 30 | |
| AADb | 39 | 15.5 | 7 | 25.9 | 46 | |
| AAD+RBVb | 25 | 9.9 | 6 | 22.2 | 31 | |
| IFN-peg+RBV | 14 | 5.6 | 2 | 7.4 | 16 | |
| IFN-peg+RBV+AADc | 16 | 6.3 | 8 | 29.6 | 24 | |
| Total | 242 | 27 | 269 | |||
| History of IFN-RBV | ||||||
| No | 184 | 73.0 | 10 | 37.0 | 194 | p<0.001 |
| Yes | 68 | 27.0 | 17 | 63.0 | 85 | |
| Exposure to IFN | ||||||
| No | 152 | 60.3 | 4 | 14.8 | 156 | p<0.001 |
| Yes | 100 | 39.7 | 23 | 85.2 | 123 | |
| Exposure to RBV | ||||||
| No | 132 | 52.4 | 0 | 0.0 | 132 | p<0.001 |
| Yes | 120 | 47.6 | 27 | 100.0 | 147 | |
| Exposure to ADD | ||||||
| No | 25 | 9.9 | 1 | 3.7 | 26 | p=0.488 |
| Yes | 227 | 90.1 | 26 | 96.3 | 253 | |
| Duration of exposure to IFN (weeks) | 16.7±29.4 | 47.2±40.1 | p=0.001 | |||
| Duration of exposure to RBV (weeks) | 17.2±30.0 | 48.9±37.7 | p<0.001 | |||
| Duration of exposure to ADD (weeks) | 12.9±7.5 | 15.7±11.6 | p=0.227 | |||
A temporal relationship could be established between treatment and the appearance of anti-RR antibodies in 17 patients (Table 4). Eight patients (1–8), previously treated with IFN-RBV, were anti-RR before treatment. In another 10 patients (18–27), it was not possible to establish a causal relationship because no previous studies were available. However, nine patients, eight of them treated with DAA, showed onset of anti-RR within three and 12 months after the end of treatment (EOT). Half of these patients had a history of treatment with IFN-RBV (including one treated with SOF-LDV) but the other half did not, and had received a combination therapy that included RBV.
Temporal relationship between the administration of anti-HCV treatment and the appearance of anti-RR.
| Patient | Anti-RR+ before treatment | Background | Treatment | Anti-RR+ post-treatment |
|---|---|---|---|---|
| 1–7 | Positive | IFNpeg-RBV | ||
| 8 | Positive | IFNpeg-RBV-TLV | Remained positive | |
| 9 | Negative | IFNpeg-RBV-SMV | SOF-LDV | 6th month |
| 10 | Negative | IFNpeg-RBV | 2D-RBV | 12th month |
| 11 | Negative | IFNpeg-RBV | 2D-RBV | 3rd month |
| 12 | Negative | IFNpeg-RBV | 2D-RBV | 3rd month |
| 13 | Negative | No | SOF-RBV | 5th month |
| 14 | Negative | No | SOF/LDV/RBV | 3rd month |
| 15 | Negative | No | 3D-RBV | 4th month |
| 16 | Negative | No | SOF-DCV-RBV | During (4th) |
| 17 | Negative | No | IFNpeg-RBV | During (11th) |
| 18–27 | Unknown | |||
RBV: ribavirin; TLV: telaprevir; SMV: simeprevir; SOF: sofosbuvir; LDV: ledipasvir; 2D: paritaprevir/ritonavir+ombitasvir; 3D: paritaprevir/ritonavir+ombitasvir+dasabuvir; DCV: daclatasvir.
The titers of anti-RR antibodies were very variable, being higher and more persistent in patients treated with IFNpeg-RBV. In contrast, the anti-RR titers in patients treated with DAA were usually very low, usually 1/80, and detectable only one to three times over less than one year. In the eight patients who had been treated with IFN-RBV and who were anti-RR-positive at baseline, the treatment had taken place 2 years before in most cases, with two patients in whom the treatment had been implemented 14 and 18 years before.
By December 2017, none of the patients had developed either AIH or evidence of other autoimmune diseases. Patients with chronic hepatitis C positive for anti-RR were not different from the rest in terms of liver disease, genotype profile, IL-28B or response to treatment (Table 3).
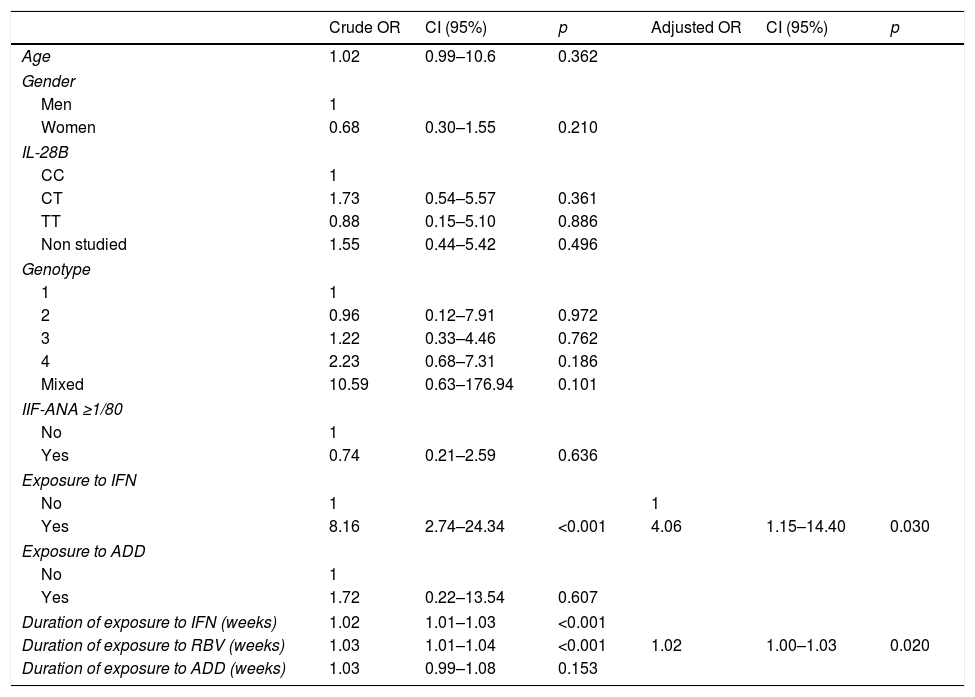
In the univariate analysis, neither the variables that did not have an independent character nor those that have not any case could be included. This obliged us to exclude “Type of treatment” (there were no patients DAA+/RR+) and the variable “Exposure to RBV” (there were no cases RBV−/RR+) (Table 5). “IFN exposure” and “Duration in weeks of exposure to IFN and RBV” were selected in the univariate analysis, and “IFN exposure” (OR: 4.06; p=0.030) and “Duration of exposure to RBV” (OR: 1.02; p=0.020) were selected in the multivariate analysis.
Univariant (left) and multivariant analysis (right).
| Crude OR | CI (95%) | p | Adjusted OR | CI (95%) | p | |
|---|---|---|---|---|---|---|
| Age | 1.02 | 0.99–10.6 | 0.362 | |||
| Gender | ||||||
| Men | 1 | |||||
| Women | 0.68 | 0.30–1.55 | 0.210 | |||
| IL-28B | ||||||
| CC | 1 | |||||
| CT | 1.73 | 0.54–5.57 | 0.361 | |||
| TT | 0.88 | 0.15–5.10 | 0.886 | |||
| Non studied | 1.55 | 0.44–5.42 | 0.496 | |||
| Genotype | ||||||
| 1 | 1 | |||||
| 2 | 0.96 | 0.12–7.91 | 0.972 | |||
| 3 | 1.22 | 0.33–4.46 | 0.762 | |||
| 4 | 2.23 | 0.68–7.31 | 0.186 | |||
| Mixed | 10.59 | 0.63–176.94 | 0.101 | |||
| IIF-ANA ≥1/80 | ||||||
| No | 1 | |||||
| Yes | 0.74 | 0.21–2.59 | 0.636 | |||
| Exposure to IFN | ||||||
| No | 1 | 1 | ||||
| Yes | 8.16 | 2.74–24.34 | <0.001 | 4.06 | 1.15–14.40 | 0.030 |
| Exposure to ADD | ||||||
| No | 1 | |||||
| Yes | 1.72 | 0.22–13.54 | 0.607 | |||
| Duration of exposure to IFN (weeks) | 1.02 | 1.01–1.03 | <0.001 | |||
| Duration of exposure to RBV (weeks) | 1.03 | 1.01–1.04 | <0.001 | 1.02 | 1.00–1.03 | 0.020 |
| Duration of exposure to ADD (weeks) | 1.03 | 0.99–1.08 | 0.153 | |||
This study shows, for the first time, that the administration of DAA in patients who have not previously been treated with IFN-RBV is unable to induce the appearance of anti-RR autoantibodies. In contrast, a history of treatment with IFN-RBV proved to be a decisive factor for the appearance of these autoantibodies. None of the 122 patients who had been treated with various combinations of DAA without ribavirin, and had no history of previous treatments, developed anti-RR, but anti-RR autoantibodies were recorded in 25.9% of those treated with DAA and who had a previous exposure to IFN-RBV. The remaining patients received RBV, or IFN-RBV, and the detection of anti-RR might always be ascribed to this drug. These results indicate that RBV is the etiologic factor most clearly associated with the development of anti-RR and underline the importance of exposure to IFN-RBV. Moreover, this study shows that it is possible to develop anti-RR autoantibodies when RBV is given associated to DAAs and without IFN, looking as if the treatment with DAA would have an immune stimulating action.
Secondly, a careful observation of the follow-up titers of anti-RR-positive patients has made us outline some general trends. The anti-RR of patients treated with DAAs seemed to develop months after the EOT, the titers did not reach high values and were short-lasting in duration, frequently less than one year. On the contrary, some patients treated with IFN-RBV were still positive more than 2 years from EOT, and occasionally much more, something ascribed to the immune stimulating action of IFN. This is in good agreement with what has been commented by other authors that a follow-up spanning up to 48 weeks is too short period of time for anti-RR clearance to take place in all patients.8–10
In third place, our study highlights some of the factors responsible for this causal relationship. The duration of treatment with RBV appears to be a relevant factor for two reasons. The first is that the rates of anti-RR autoantibodies in patients previously exposed to IFN-RBV are the highest found in this study. This would indicate that successive exposures to IFN-RBV may affect the development of anti-RR antibodies, a factor that explains a positive selection of REL or NR to treatment in previous studies.7–9,11 Secondly, and no less important, the result of the multivariate analysis indicates that the time of exposure to RBV is an independent factor. Moreover, the statistical analysis also confirmed the importance of IFN, which would explain the maintenance of the highest titers of anti-RR antibodies. The statistical treatment of the data slightly undermined two of the most important conclusions of the study. First, the lack of a single case of anti-RR in patients treated with only DAA prevented the inclusion of the “Type of treatment” variable (Table 3) in a multivariate analysis. The same occurred with “Exposure to RBV”, since none of the patients not taking RBV was anti-RR-positive. These two strong associations cannot be properly highlighted due to the lack of anti-RR in patients taking only DAA. These cases are the rarest, and a much larger series would have been required to find patients with these characteristics.
Some authors have associated the presence of anti-RR in patients with chronic hepatitis C more with the immune background of this disease than with the treatment.12 In a population-based study, Shaikh et al. confirmed the presence of these autoantibodies in 39 of 4699 subjects (0.74%) of whom 38 were negative anti-HCV.13 In many cases, the presence of anti-RR was associated with low titers, the existence of medication and/or associated autoimmune diseases. In our study, three patients with autoimmune diseases and anti-RR autoantibodies and who were negative for HCV were also detected. These data define at least three different situations in which the presence of anti-RR should be considered: medication with anti-RR inducing-capacity, autoimmunity and treatment of chronic hepatitis C with IFN-RBV. However, only in the case of treatment of hepatitis C with IFN-RBV can this phenomenon be considered frequent and predictable. The positivity of anti-RR in this last subset of patients raises another concern, i.e., the risk of these patients to develop an autoimmune disease or the development of AIH after treatment of HCV infection. However, none of these risks have appeared up to now in any patient and the anti-RR seroconversion appears as a temporary, although sometimes long-lasting, loss of immunological tolerance breakdown.
In patients with hepatitis C treated with DAA, with previous exposure to IFN-RBV, there must be a mechanism that explains the appearance of anti-RR. The aggregation of IMPDH-2 (or CTPS-1) by the action of one of the multiple agents used in experimental conditions (ribavirin, mycophenolic acid, acivicin, DON, under cellular deprivation conditions of gutamine, serine, guanine, or by inhibiting dihydrofolate-reductase with methotrexate and aminopterin), and its subsequent disengagement (after adding hypoxanthine or guanosine14), indicates that the formation of RR may be a mechanism effected by cells to control of their enzymatic activity.12,15 It is tempting to speculate that, in some patients with a history of IFN-RBV, DAAs would create the cellular conditions for IMPDH-2 to re-assemble, stimulating the immune system and inducing a new flare-up of autoantibodies. Obviously, attempting to outline a mechanism of this new aggregation remains far beyond the scope of the study.
In 2016, among the difficult-to-treat patients, those infected by genotype 3 remained as candidates for receiving RBV combined with DAAs. However, in July 2016 we had the last patient treated with RBV (SOF-DCV-RBV) and, since then, new patients with this genotype have been treated with Sofosbuvir-Velpatasvir with/without Voxilaprevir, new therapeutic combinations for infections of genotype 3.15 This marked the end of the use of RBV in the treatment of chronic hepatitis C and prevented us from obtaining new data. Hepatitis E patients, in whom ribavirin administered off-label in transplant patients seems to have therapeutic activity,16 may give us another option to extend our knowledge of this drug.
In conclusion, patients treated only with DAA have no risk of developing autoantibodies against Rods & Rings. However, those patients treated with DAA bear some risk of developing anti-RR if treated with RBV or if treatment with IFN-RBV has been an earlier event. The ultimate meaning of this transient loss of immune tolerance is unknown.
Ethical approvalData were collected and samples were obtained after approval of the protocol by the regional committee of clinical trials, CEI Girona, Code RODS and RINGS. Patients’ informed consent were obtained.
FundingNone declared.
Conflict of interestThe authors reported no conflict of interests in relation to this study.