Olmesartan is a potential cause of drug-induced enteropathy. Two case reports suggest the association of olmesartan with the development of drug induced liver injury (DILI).1,2 We present a case of hypertransaminasemia showing temporal association between exposure to olmesartan and onset of clinics, recovery after withdrawal and several episodes of recurrence, the first of them after reexposure to olmesartan. Autoimmunity tests and liver biopsies suggested an autoimmune mechanism to explain this phenomenon. Differencial diagnosis between DILI and autoimmune hepatitis was difficult to carry out, but the second episode of recurrence and the evolution after corticosteroid therapy supported the diagnosis of chronic autoimmune hepatitis triggered by olmesartan.
A 61-year old Caucasian male who works as a doctor presented in the Emergency Department of our centre in February 2017 with a 1-month history of vespertine febricula, malaise, fatigue, anorexia, arthromyalgia and weight loss. The patient had no significant medical history except for arterial hypertension and a choledocal cyst operated in 2007 with Roux-en-Y hepaticojejunostomy and cholecystectomy. There was no familial history of chronic liver diseases or significant alcohol intake. He was under treatment with olmesartan 10mg once daily. Laboratory tests revealed 50% of activated lymphocytes, absence of coagulopathy, elevation of liver enzymes [aspartate aminotransferase 504U/L (N<37), alanine aminotransferase 568U/L (N<41), alkaline phosphatase 143U/L (N<129), gamma-glutamyl transpeptidase 135U/L (N<50)] with total bilirubin 0.9mg/dL (N<1.0mg/dL) and elevation of lactate dehydrogenase 357U/L (N<235U/L), C-reactive protein 75.6mg/L (N<5.0mg/L) and ferritin 1096ng/mL (N<300ng/mL); with no alterations in the rest of iron tests. Initially presumed to be a part of a mononucleosis syndrome, viral serologies (including hepatitis A, B, C, D and E viruses, cytomegalovirus, Ebstein-Barr virus and HIV) resulted negative. Abdominal ultrasonography was unremarkable except for previously known cholecystectomy. In the setting of this clinical presentation the patient had normal levels of blood pressure so he decided to withdraw treatment with olmesartan. Three months later the patient remained asymptomatic, with normal laboratory parameters except for mild elevation of aspartate aminotransferase (48U/L) and lactate dehydrogenase (286U/L). The patient refused to conduct further study and he was later lost to follow-up.
The patient consulted again in April 2018 because of clinical worsening. He declared having been taking olmesartan during the prior 6 months. Laboratory tests showed elevation of total bilirubin (1.5mg/dL), aspartate aminotransferase (684U/L), alanine aminotransferase (705U/L), alkaline phosphatase (126U/L), gamma-glutamyl transpeptidase (244U/L), lactate dehydrogenase (315U/L), C-reactive protein (22.20mg/L) and ferritin (1320ng/mL). Study of Wilson's disease, hereditary hemochromatosis and alpha-1-antitrypsine deficiency were all negative. Serum immunoglobulin IgA, IgG and IgM were normal. Determination of anti-smooth muscle antibodies was positive (titre 1:80). Antinuclear, anti-mitochondrial and anti-liver kidney microsomal antibodies were negative. Even though our team suggested to perform a liver biopsy, the patient remained reluctant to it.
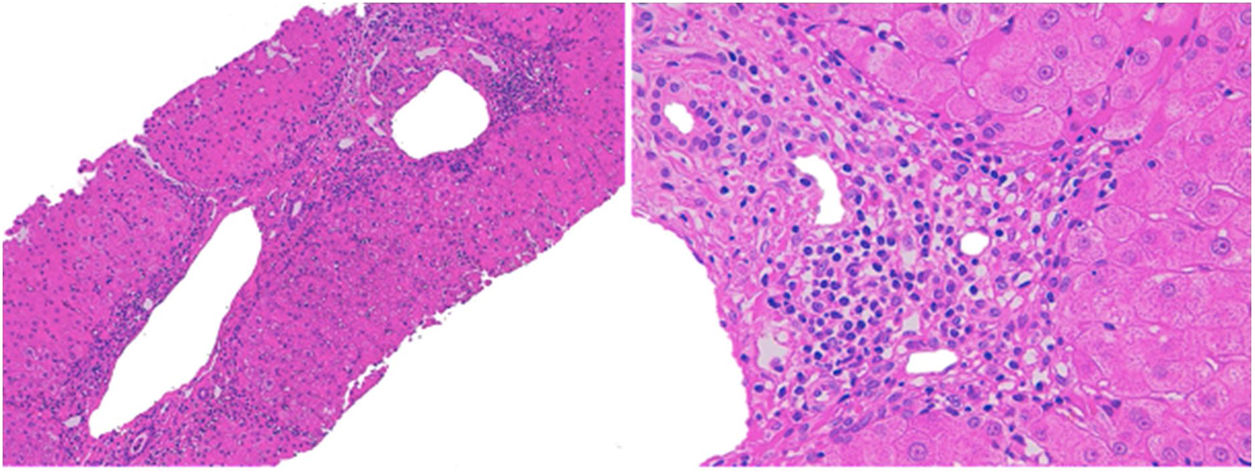
We decided to discontinue treatment with olmesartan. Symptoms and laboratory parameters improved seven days and one month after withdrawal, respectively. Two months later the patient presented increase of IgG (1620mg/dL [N 690–1400mg/dL]). Liver biopsy was performed this time with the patient's consent, which showed septal fibrosis and periportal hepatitis with the presence of lymphoplasmocytic and eosinophilic infiltration, without significant steatosis (Fig. 1). These findings suggested an autoimmune hepatitis although the presence of eosinophils was more characteristic of a drug-induced mechanism. Follow-up 4 months after withdrawal of olmesartan showed no remarkable alterations in laboratory parameters, including liver enzymes and serum immunoglobulins. Due to the improvement in laboratory parameters, the patient refused to receive any treatment. After 12 months of follow-up, he presented an increase of aspartate aminotransferase (221U/L), alanine aminotransferase (190U/L) and IgG (1670mg/dL). We decided then to begin treatment with oral prednisone 40mg daily. 4 weeks after start of corticosteroid therapy liver parameters were normal again, which allowed corticosteroid tapering. After subsequent corticosteroid suspension, the patient remains currently in complete remission.
The diagnostic of DILI was supported by the temporal association between exposure to olmesartan and liver damage, as well as recurrence after reexposure. Casualty assessment was performed using the CIOMS/RUCAM score which was 7 (probable). Other concomitant diseases or potential causes of liver injury were reasonably excluded.3 However, the presence of further episodes of recurrence, serum anti-smooth muscle antibodies, elevated IgG and response to corticosteroid therapy corresponded better to the diagnosis of a chronic autoimmune hepatitis. The histological findings were compatible to both entities: the presence of eosinophils is associated with cases of DILI, but periportal hepatitis and the presence of plasmocytic cells are typical of autoimmune hepatitis. Besides, the presence of septal fibrosis is more consistent to a chronic process, such as autoimmune hepatitis.4 We concluded therefore that a chronic autoimmune hepatitis triggered by olmesartan was the most likely diagnosis.
There are already two cases that have reported an association between olmesartan and DILI. In one of them the appearance of non-alcoholic steatohepatitis might be a consequence of an indirect effect of olmesartan-induced sprue-like enteropathy.1 Barge et al.2 reported the presence of chronic hepatitis with septal fibrosis along with elevation of antinuclear antibodies and IgG that suggested the presence of an autoimmune-like mechanism, regressive after olmesartan withdrawal and with no association with sprue-like enteropathy. Our data seem to be more consistent with an underlying autoimmune mechanism. Many drugs have been associated with drug-induced autoimmune hepatitis. Suspected cases should be evaluated thoroughly to exclude the possibility of idiopatic autoimmune hepatitis, including causality assessment, serology and liver biopsy. In cases who do not show complete remission after drug cessation, it is reasonable to begin corticosteroid therapy in order to alleviate symptoms and speed recovery.5,6 Future follow-up with closely monitoring and determination of HLA could help clarify this case.
Conflict of interestThe authors declare no conflict of interest.