To assess the effectiveness and safety of cap-assisted endoscopic resection and the usefulness of endoscopic ultrasonography (EUS) for managing small rectal subepithelial tumors (SETs).
Patients and methodsPatients with small rectal SETs≤10mm in diameter were enrolled in this study at our hospital from October 2014 to December 2017. First, EUS was performed to evaluate the lesions. Then, cap-assisted endoscopic resection was performed by suctioning the SET into a transparent cap, ligating with a metal snare and then resecting the tumor. The wound was closed using endoclips if necessary.
ResultsForty patients were enrolled in the study. EUS showed lesions originating from muscularis mucosa or submucosa with an average diameter of 5.4×3.1mm. The en bloc resection rate was 85.0% obtained by cap-assisted endoscopic resection, with a mean total procedure time of 17.6min. No immediate perforation happened. Immediate bleeding occurred in five patients; all cases were managed successfully by endoscopy. No delayed bleeding was observed. Pathology examination showed that 70.0% of the lesions were neuroendocrine tumors (G1). One case of recurrence was seen in follow-up; it was managed successfully by endoscopic submucosal dissection. There was no tumor recurrence in a median follow-up period of 41 months in the remaining 39 patients.
ConclusionsMost small rectal SETs arising from the muscularis mucosa or submucosa are neuroendocrine tumors and require proper treatment. Cap-assisted endoscopic resection is simple, effective and safe for resecting such lesions, and EUS is useful for case screening.
Evaluar la eficacia y la seguridad de la resección endoscópica asistida por capuchón y la utilidad de la ultrasonografía endoscópica (USE) para el tratamiento de pequeños tumores subepiteliales (TSE) rectales.
Pacientes y métodosLos pacientes con TSE rectales pequeños ≤10 mm de diámetro se enrolaron en este estudio en nuestro hospital desde octubre de 2014 hasta diciembre de 2017. Primero, se realizó una USE para evaluar las lesiones. Luego, se realizó una resección endoscópica asistida por capuchón aspirando el TSE en un capuchón transparente, ligándolo con una asa metálica de polipectomía y luego resecando el tumor. La herida se cerró usando endoclips, si ello era necesario.
ResultadosCuarenta pacientes fueron enrolados en el estudio. La USE mostró lesiones originadas en la muscularis mucosae o submucosa con un diámetro promedio de 5,4 × 3,1 mm. La tasa de resección en bloque fue del 85,0% obtenida mediante resección endoscópica asistida por capuchón, con un tiempo total medio de procedimiento de 17,6 min. No se produjo ninguna perforación en el momento. Se produjo una hemorragia inmediata en cinco pacientes; todos los casos se trataron con éxito mediante una endoscopia. No se observó ningún retraso en el sangrado. El examen patológico mostró que el 70% de las lesiones eran tumores neuroendocrinos (G1). En el seguimiento se observó un caso de recurrencia, el cual se trató con éxito mediante una disección endoscópica de la submucosa. No hubo recurrencia de tumores en un período de seguimiento medio de 41 meses en los 39 pacientes restantes.
ConclusionesLa mayoría de los TES rectales pequeños que surgen de la muscularis mucosae o submucosa son tumores neuroendocrinos y requieren de un tratamiento adecuado. La resección endoscópica asistida por capuchón es simple, eficaz y segura para resecar tales lesiones y la USE es útil para la detección de casos.
Rectal subepithelial tumors (SETs) are usually asymptomatic and accidentally discovered by colonoscopy.1,2 However, common colonoscopy can only supply limited information for assessment of these lesions. Therefore, further evaluation by other cross-sectional imaging modalities may be needed for choosing appropriate management. It has been reported that endoscopic ultrasonography (EUS) has advantages in evaluation of tumor size and invasion depth of SETs.3–5
Several resection techniques such as endoscopic mucosal resection (EMR), EMR with circumferential incision, EMR with a ligation device (EMRL), EMR using a cap (EMRC), endoscopic submucosal dissection (ESD) and transanal endoscopic microsurgery (TEM) have been reported for treatment of rectal SETs.3,4,6–9 Every method has its disadvantages. For example, ESD is very effective, but it's skill-demanded and time-consuming for endoscopic freshman. Hence it cannot be applied in preliminary hospitals. In 1996, Kajiyama et al.10 reported 38 cases in which a transparent cap was used to remove small submucosal GI tumors, with few adverse events. The tumor was first aspirated into the cap, grasped at its base with a snare, removed by electrocautery and then retrieved by aspirating into the cap. Our group has applied this method in small submucosal tumors originating from the muscularis propria of the gastric fundus and showed that it was a simple and safe technique and much easier to be applied than ESD.11
The main aim of this study was to evaluate the effectiveness and safety of cap-assisted endoscopic resection technique for small rectal SETs originating from muscularis mucosa or submucosa by the guidance of EUS. The second aim was to investigate the pathological types of such SETs.
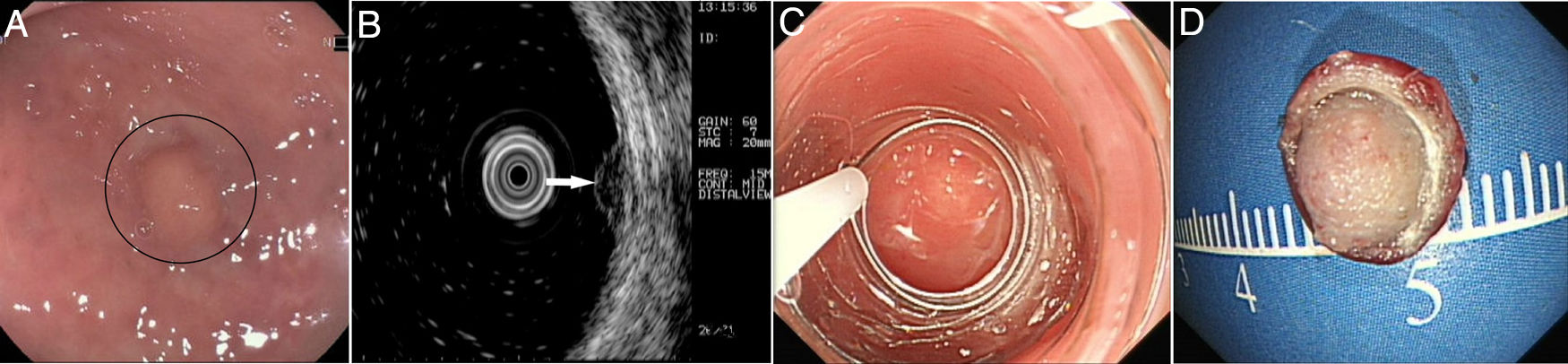
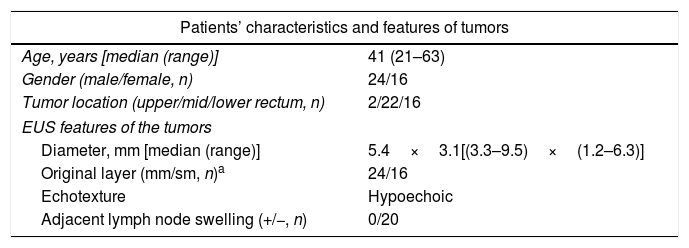
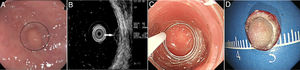
Patients and methodsPatientsFrom October 2014 to December 2017, 40 consecutive patients with small rectal SETs (≤10mm in diameter) originating from the muscularis mucosa or submucosa were enrolled in this observational single-arm case series. The invasion depth and size of the SETs were defined by preoperative EUS combined with colonoscopy (Fig. 1a, b). Chest & abdominal CT and pelvic cavity MRI scan were also performed to exclude metastasis. The suitable patients then underwent cap-assisted endoscopic resection at the department of gastroenterology of our hospital after written informed consents were obtained. Exclusion criteria were: (1) patients aged<18 or >65 years; (2) patients with coagulopathy; (3) those with severe general condition, such as heart failure, renal failure, uncontrolled hypertension or diabetes mellitus; (4) those having mental diseases with no or limited autonomy; (5) pregnancy or lactation; and (6) those who did not provide written informed consents. The study protocol was adhered to the Declaration of Helsinki and approved by the human ethics committee of our hospital.
EUSThe patients were instructed to clean their bowels by oral lavage beforehand. During the EUS examination, the patient was in a common left lateral decubitus position, which was adjusted if necessary to achieve optimal observation of the area of interest. The procedure was performed one to seven days before endoscopic resection by using a 15MHz miniprobe (Fujinon SP-702, Japan) after water infusion to immerse the lesion. The diameter, originating layer, echotexture and adjacent lymph node swelling were recorded.
Cap-assisted endoscopic resectionCap-assisted endoscopic resection was performed by two endoscopists who were experienced in ESD and/or EFTR. The patients were instructed to clean their bowels by oral lavage beforehand and were administrated intravenously with prophylactic antibiotic (second-generation cephalosporins) two hours before resection. Carbon dioxide (CO2) insufflation was employed using the UCR Endoscopic CO2 Regulation Unit (Olympus, Tokyo, Japan) during the procedure. Endoscopic examination (GIF-XQ260, Olympus) of the rectum was first performed to confirm the location and appearance of the tumor, and then the mucosa overlying the tumor was marked with the tip of a snare (SD-7P-1, Olympus). This kind of snare was crescent with a ring diameter of 25mm.
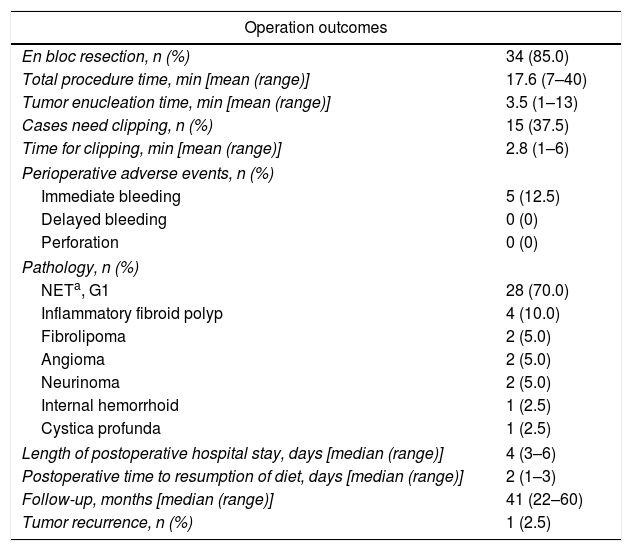
A transparent cap (MH-593, Olympus) was then attached to the forward-view endoscope. The cap was straight and 12mm long with an outer diameter of 12.9mm. After the endoscope was inserted into the rectum and located the tumor, a small piece of rectal mucosa was slightly suctioned. Then the snare was inserted through the endoscopic working channel and fixed around the inner circumference of the cap (Fig. 1c). The tumor was then suctioned into the cap and the snare was closed. After confirming appropriate placement of the snare, both the tumor and the overlying mucosa were resected by electrocautery (Endocut Q, effect 2, VIO 200D; ERBE, Tübingen, Germany), aspirated into the cap and then sent for pathological examination. Endoscopic examination was repeated without the cap in order to evaluate the wound carefully to make sure if there existed perforation or residual tumor tissues. Visible bleeding was carefully coagulated. If the defect was too whitish or suspect perforation existed, it would be closed by using endoclips (EZ-CLIP, HX-110QR, Olympus or Resolution).
Postoperative managementThe patients were fasted for at least one day. Any possible signs of bleeding or perforation were carefully monitored. On postoperative day 2, the patients were started on no residue diet and switched gradually to semi-solid and solid food. Patients were advised to follow up at the outpatient department of gastroenterology with EUS, chest x-ray and abdominal ultrasonography every six months in the first two years. From the third year postoperatively, follow-up annually was suggested. Pelvic cavity MRI scan was also performed if necessary.
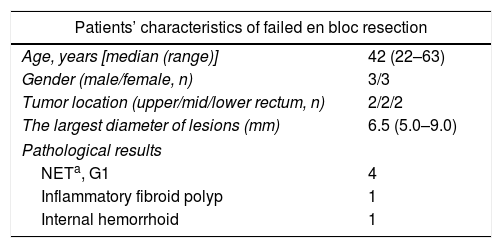
PathologyThe resected tumors were placed on a plastic foam board with a paper ruler and the tumor margins were carefully examined (Fig. 1d). All the resected specimens were fixed in formalin, embedded in paraffin, cut into 3-μm-thick sections, and stained with hematoxylin and eosin (HE). Immunohistochemical analysis was performed if necessary according to the pathologists. Pathological examination was performed by qualified pathologists and any disagreement was resolved by consensus.
Statistical analysisQuantitative parameters were expressed as means or medians with ranges, while qualitative parameters were expressed as numbers and percentages or frequencies. Quantitative data were analyzed using t-test, while qualitative data were analyzed using chi-square test.
ResultsPatients’ characteristics and features of the tumorsA total of 40 patients including 24 men and 16 women with a median age of 41 years (ranging from 21 to 63 years) were enrolled in this study.
According to the distance between the tumor and the anal edge, rectal SETs were divided into upper rectal SETs (distance of 11–15cm), middle rectal SETs (distance of 6–10cm), and lower rectal SETs (distance of 3–5cm). Among the 40 small rectal SETs, 22 (55.0%) were located at the middle rectum, 16 (40.0%) at the lower rectum and 2 (5.0%) at the upper rectum. The average tumor size measured by EUS was 5.4mm×3.1mm. EUS also showed that 60.0% (24/40) of the tumors originated from the muscularis mucosa and all the lesions were hypoechoic. No swollen lymph node was detected by EUS or pelvic cavity MRI scan. No metastatic lesion was found by other examinations. The characteristics of the patients and the features of the tumors are shown in Table 1.
Patients’ characteristics and features of the tumors.
| Patients’ characteristics and features of tumors | |
|---|---|
| Age, years [median (range)] | 41 (21–63) |
| Gender (male/female, n) | 24/16 |
| Tumor location (upper/mid/lower rectum, n) | 2/22/16 |
| EUS features of the tumors | |
| Diameter, mm [median (range)] | 5.4×3.1[(3.3–9.5)×(1.2–6.3)] |
| Original layer (mm/sm, n)a | 24/16 |
| Echotexture | Hypoechoic |
| Adjacent lymph node swelling (+/−, n) | 0/20 |
All the cap-assisted endoscopic resection procedures were successfully finished with an average total operation time of 17.6min (range 7–40min) (Table 2), and the mean enucleation time was 3.5min (range 1–13min). The en bloc resection rate was 85.0% (34/40).
Outcomes of patients treated by cap-assisted endoscopic resection.
| Operation outcomes | |
|---|---|
| En bloc resection, n (%) | 34 (85.0) |
| Total procedure time, min [mean (range)] | 17.6 (7–40) |
| Tumor enucleation time, min [mean (range)] | 3.5 (1–13) |
| Cases need clipping, n (%) | 15 (37.5) |
| Time for clipping, min [mean (range)] | 2.8 (1–6) |
| Perioperative adverse events, n (%) | |
| Immediate bleeding | 5 (12.5) |
| Delayed bleeding | 0 (0) |
| Perforation | 0 (0) |
| Pathology, n (%) | |
| NETa, G1 | 28 (70.0) |
| Inflammatory fibroid polyp | 4 (10.0) |
| Fibrolipoma | 2 (5.0) |
| Angioma | 2 (5.0) |
| Neurinoma | 2 (5.0) |
| Internal hemorrhoid | 1 (2.5) |
| Cystica profunda | 1 (2.5) |
| Length of postoperative hospital stay, days [median (range)] | 4 (3–6) |
| Postoperative time to resumption of diet, days [median (range)] | 2 (1–3) |
| Follow-up, months [median (range)] | 41 (22–60) |
| Tumor recurrence, n (%) | 1 (2.5) |
Immediate bleeding occurred in 5 (12.5%) patients, which was managed successfully by using coagrasper (Olympus, Japan). No delayed bleeding or perforation was observed in any patient. Endoclips were used in 15 (37.5%) patients to close the defect because it appeared too whitish. The mean clipping time was 2.8min (range 1–6min).
All patients resumed a no-residue diet within a median of 2 days (range 1–6 days) after the procedure and gradually changed to a normal diet. Patients were discharged from hospital 4 days (range 3–6 days) after the operation. Local recurrence was found in 1 case 6 months after endoscopic resection, which was successfully resected by ESD. There was no tumor recurrence during a median follow-up period of 41 months (range 22–60 months) in the remaining 39 patients. No metastasis was observed during the follow up period.
Pathological resultsThirty-four of the resected specimens had negative lateral and vertical margins, defined as en bloc resection, while the remaining six specimens’ vertical or lateral margins could not be evaluated because of heavy electrocautery which was defined as ‘indeterminate margins’. Histologically, 70.0% (28/40) of the tumors were neuroendocrine tumors (NETs, G1), and the remaining pathological results included inflammatory fibroid polyp, fibrolipoma, angioma, internal hemorrhoid, cystica profunda and neurinoma, as shown in Table 2. The success rate of en bloc resection was significantly related with location of the lesions (P=0.002), but not related with the largest diameter of the lesion, patients’ age, gender or pathological results of the tumors (P>0.05). The characteristics of failed en bloc resection lesions are shown in Table 3.
Characteristics of failed en bloc resection lesions.
| Patients’ characteristics of failed en bloc resection | |
|---|---|
| Age, years [median (range)] | 42 (22–63) |
| Gender (male/female, n) | 3/3 |
| Tumor location (upper/mid/lower rectum, n) | 2/2/2 |
| The largest diameter of lesions (mm) | 6.5 (5.0–9.0) |
| Pathological results | |
| NETa, G1 | 4 |
| Inflammatory fibroid polyp | 1 |
| Internal hemorrhoid | 1 |
With modification of the endoscopic equipment, more and more SETs are detected in routine colonoscopy. In this study we demonstrated that the cap-assisted endoscopic resection technique was a simple and safe method for the effective resection of small rectal SETs (≤10mm in diameter) originating from the muscularis mucosa or submucosa confirmed by preoperative EUS.
During routine colonoscopy SETs are usually seen as wide-based sessile protruded lesions with an almost normal mucosal surface.12 It is known that EUS is very useful in assessment of tumors in GI tract, especially in exhibiting the diameter and originating layer of the tumours.4,12,13 The EUS findings are helpful for differential diagnosis in most cases. Therefore, we can choose resection or follow-up strategy reasonably. For example, colorectal lipoma or cyst do not need further management but only follow-up by EUS or colonoscopy unless they complicate with bleeding, intestinal obstruction or other adverse events.14 However, carcinoid tumor (NET, G1) need further intervention by endoscopic or surgical resection due to its potential malignancy.15
In the present study, all the lesions were found by routine colonoscopy at local hospital or our hospital. EUS was performed for every lesion, which showed that all tumors were hypoechoic and originated from the muscularis mucosa or submucosa. So diagnosis of suspected NET, G1 (carcinoid tumor) was made. The postoperative pathological results showed that EUS got an accuracy of 70.0% (28/40). Three of them were inflammatory fibroid polyps (IFPs) proved by pathology. IFPs are rare benign tumors in GI tract, some of which mimick SETs. And for EUS, they can also be hypoechoic and originate from the muscularis mucosa or submucosa,16,17 so misdiagnosis may be made. An interesting case in this study needed to be pointed out is an internal hemorrhoid. It was hypoechoic and originated from the muscularis mucosa in EUS view. Obvious bleeding occurred during the resection and the hemostasis procedure cost eight minutes. This case reminds us that SETs located near the anal edge may be untypical hemorrhoids and we should be cautious in resecting this kind of lesions.
Many kinds of technique have been reported for the treatment of rectal SETs, such as EMR, EMR with circumferential incision, EMRL, EMRC, ESD and TEM. However, each method has its advantages and disadvantages.18,19 Cap-assisted endoscopic resection is a simple procedure that can be performed quickly and effectively for small GI SETs. It has advantages over ESD and TEM, as it includes only three steps, suction, cut and clip (if necessary), which is less time-consuming and less demanding of skills. In the present study, the average operation time was only 17.6min (range 7–40min). Thirty-four of forty cases (85.0%) obtained en bloc resection, similarly to the report of ESD.20 Immediate bleeding occurred in 5 cases (12.5%), one of which was an internal hemorrhoid case and all cases were managed successfully by endoscopy. No delayed bleeding or perforation was observed. The complication rate was higher than that of ESD reported by Chen et al.20 and it may be related with our small sample size. One locally recurrent case was observed during follow-up, which was then successfully resected by ESD. In this case, the previous endoscopic therapy didn’t get negative margins and it may be related with the local recurrence. By statistical analysis, we can see the failed en bloc resection was significantly associated with location of lesions but not the size. The lesions in the upper rectum were both located behind the fold, so they were difficult to be identified and might be missing when suctioned. But this result needs further verification because the small sample size of our study.
Compared with ESD, there is one limitation to cap-assisted endoscopic resection as reported in our previous study.11 The transparent cap available for the procedure is not over 10mm in inner diameter, suggesting that cap-assisted endoscopic resection is not suitable for SETs of >10mm in size. Therefore, cap-assisted endoscopic resection is recommended only for small rectal SETs. However, cap-assisted endoscopic resection is more time-saving and simpler, less skill-demanded than ESD and it can be carried out at most endoscopy centers.
There were limitations to this study. First, the margins of six samples could not be evaluated because of heavy electrocautery. Hence a long follow-up period of more than one year is needed to confirm a complete removal of the tumor. Second, the sample size was small and it was a single center observational study. So randomized controlled multi-center studies comparing cap-assisted endoscopic resection with ESD or other endoscopic procedures may be needed to further assess the efficacy and limitations of cap-assisted endoscopic resection in the future.
In conclusion, most small rectal SETs originating from the muscularis mucosa or submucosa are neuroendocrine tumors which need proper therapy. Moreover, cap-assisted endoscopic resection may be a simple, effective and safe technique for such cases and EUS is useful for case screening. Prospective, randomized controlled studies are needed to assess the indications for cap-assisted endoscopic resection and outcomes of the patients after this procedure in the future.
FundingThis work was supported by the Open Subject of Guangdong Provincial Key Laboratory of Gastroenterology (2017).
Conflict of interestNone.