Despite advances in imaging techniques, in many cases they are insufficient to establish the diagnosis of pancreatic cystic lesions (PCL). There are few publications in our setting that evaluate the combination of several methods obtained by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). The aim of the study was to evaluate the overall utility of EUS-FNA in the diagnosis of PCL.
Material and methodsRetrospective study based on a database updated prospectively of a cohort of patients referred for EUS-FNA due to PCL detected in an imaging test. The sensitivity, specificity and diagnostic yield of carcinoembryonic antigen (CEA), cytology and viscosity were studied to detect mucinous lesions.
ResultsFrom November 2013 to April 2018, 122 EUS were performed for PCL. EUS-FNA was performed in 94/122 (77%) and 21/122 (17.2%) patients were operated on. We included 33/122 patients who had diagnostic confirmation by histology, imaging (serous cyst with typical pattern) or clinical evolution. The study of the ROC curve determined the cutoff point ≥419ng/ml to differentiate mucinous/non-mucinous cystic lesions. The diagnostic yield of CEA was 87.5% (21/24), cytology 81.8% (27/33) and viscosity 84.4% (27/32). The three parameters in combination obtained the best result (30/33, 90.9%).
ConclusionThe combination of CEA analysis, cytology and viscosity of pancreatic fluid obtained by EUS-FNA increases the performance in the diagnosis of mucinous pancreatic cystic lesions, with it being greater than 90%.
A pesar de los avances en las técnicas de imagen, en muchos casos, son insuficientes para establecer el diagnóstico de las lesiones quísticas pancreáticas (LQP). Son escasas las publicaciones en nuestro medio que evalúan la combinación de varios métodos obtenidos mediante la punción aspirativa con aguja fina con ultrasonografía endoscópica (USE-PAAF). El objetivo del estudio fue evaluar la utilidad global de la USE-PAAF en el diagnóstico de las LQP.
Material y métodosEstudio retrospectivo a partir de una base de datos actualizada prospectivamente de una cohorte de pacientes remitidos para USE-PAAF por LQP. Se estudió la sensibilidad, especificidad y el rendimiento diagnóstico del antígeno carcinoembrionario (CEA), la citología y la viscosidad para detectar lesiones mucinosas.
ResultadosDesde noviembre de 2013 a abril del 2018 se realizaron 122 USE por LQP. Se realizó USE-PAAF en 94/122 (77%) y se intervinieron 21/122 (17.2%) pacientes. Se incluyeron 33/122 pacientes que tuvieron confirmación diagnóstica mediante histología, imagen (quiste seroso con patrón típico) o evolución clínica. El estudio de la curva ROC determinó el punto de corte ≥ 419ng/ml para diferenciar lesión quística mucinosa/no mucinosa. El rendimiento diagnóstico del CEA fue del 87.5% (21/24), de la citología del 81.8% (27/33) y de la viscosidad del 84.4% (27/32). Los tres parámetros en combinación obtuvieron el mejor resultado (30/33, 90.9%).
ConclusiónLa combinación del análisis del CEA, citología y viscosidad del líquido pancreático obtenido mediante USE-PAAF aumenta el rendimiento en el diagnóstico de lesiones quísticas pancreáticas mucinosas, siendo superior al 90%.
The diagnosis of pancreatic cystic lesions (PCL) has increased in recent years. One explanation for the higher detection rates and frequency could be the increasingly widespread use of imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI). Their prevalence also increases with age, with approximately 10% of patients over 70 years of age developing PCL.1 Most of these lesions are asymptomatic and are incidental findings. PCL are reportedly found in up to 14% of patients having an MRI for reasons unrelated to the pancreas.2 Endoscopic ultrasound (EUS) can also identify small lesions in the pancreas. A recent Spanish prospective study reports a PCL detection rate of 22% when performed for an indication unrelated to the pancreas.3
PCL make up a very diverse group of lesions. They range from inflammatory, such as pseudocysts, to neoplastic, with a very different prognosis and requiring a different therapeutic approach. PCL are classified as mucinous or non-mucinous. Mucinous PCL include intraductal papillary mucinous neoplasms (IPMN) and mucinous cystic neoplasms (MCN) and are important because they are pre-malignant lesions.4
Despite the advances in imaging techniques, in many cases they are insufficient to establish the diagnosis, making the diagnostic-therapeutic approach challenging. This is because PCL have a broad clinical spectrum and it can be difficult to establish the prognosis of malignancy, with similar results obtained by CT, MRI and EUS (without fine-needle aspiration [FNA]).5
The advantage of EUS over other imaging techniques is the fact that FNA can be performed to obtain samples for study, which could provide increased diagnostic efficiency.6 The accuracy of EUS-FNA in solid pancreatic lesions has been widely demonstrated (around 90%).7 In contrast, the accuracy of cytology in cystic lesions is lower (around 50%),7,8 making its utility more subject to debate. Due to the poor accuracy of cytology, a number of studies have analysed different parameters in the cyst fluid (viscosity, tumour markers, etc.).9 Performing one single determination has its limitations, but few studies have assessed using a combination of methods.
In view of the lack of studies in our setting assessing the overall utility of EUS-FNA in PCL (macroscopic, cytological and biochemical study of the fluid obtained), and the limited utility of each of these methods individually, it was decided to conduct this study with the aim of assessing the utility of EUS-FNA in the diagnosis of PCL by combining three tests (viscosity, cytology and carcinoembryonic antigen [CEA]).
Patients and methodsPatients and endoscopic ultrasound procedureRetrospective study based on a database of prospective inclusion of patients with an undetermined PCL finding by imaging test (CT and/or MRI), and the use of EUS±FNA in a tertiary hospital from November 2013 to April 2018. The study was approved by the hospital’s ethics committee and all patients signed an informed consent form.
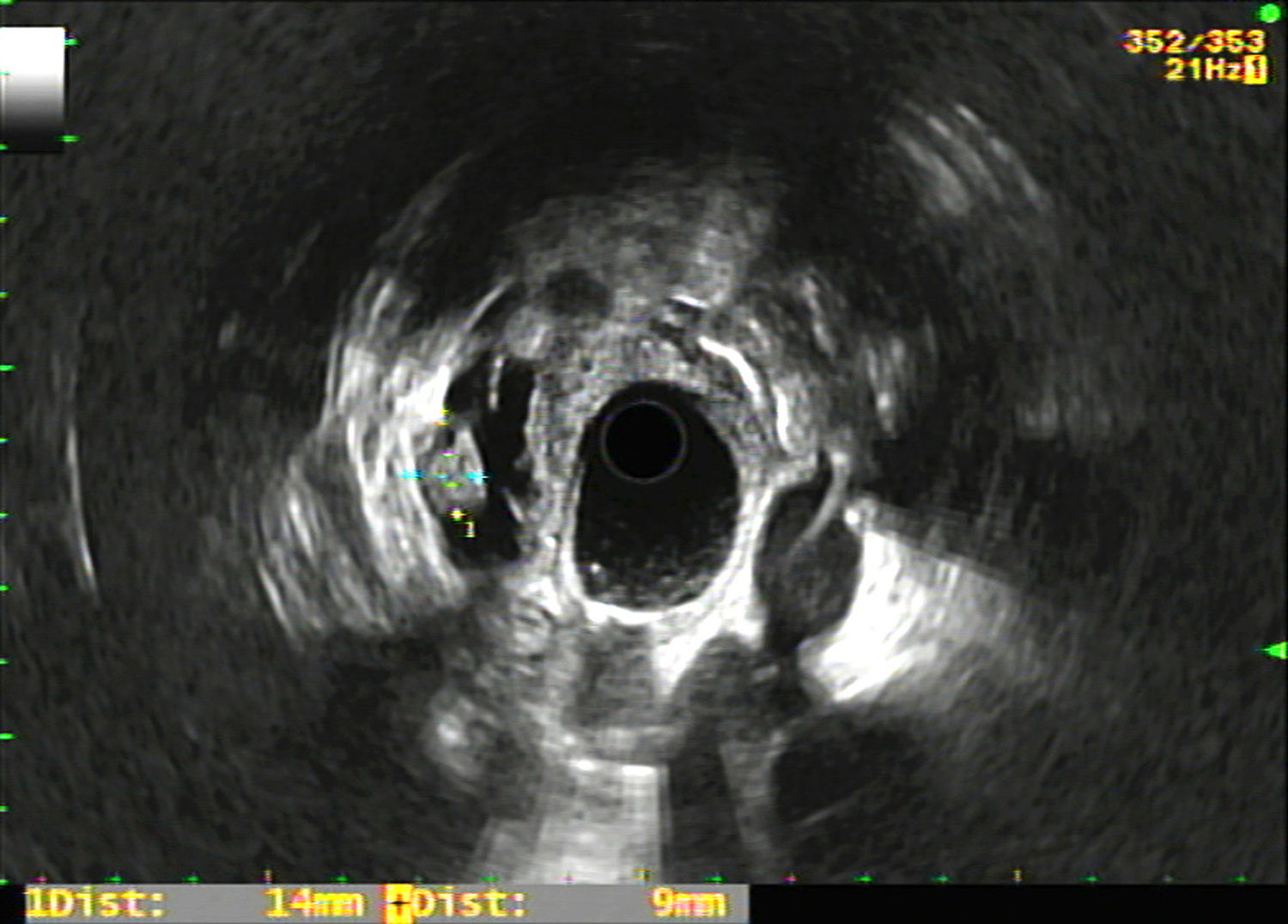
The registry included epidemiological variables (age, gender), pharmacological variables (treatment with antiplatelet agents and/or anticoagulants), size and location of the PCL, morphological characteristics in the EUS of the PCL (Fig. 1) and the rest of the pancreas (existence of septation, mural nodules, solid portion, calcification, wall thickening, communication with the pancreatic duct, dilation of the Wirsung duct and parenchymal and/or duct findings consistent with or suggestive of chronic pancreatitis according to the Rosemont classification10), puncture results (macroscopic appearance of the cyst fluid, CEA level and cytology study), complications deriving from the EUS-FNA, surgical intervention and histology result, as well as radiological and clinical follow-up of the non-operated patients. Complications and patient outcomes were recorded by consulting the electronic medical records.
The EUS was performed with a radial endoscopic ultrasound (GF-UE160-AL5, Olympus America Inc., Melville, NY, USA) followed by a linear endoscopic ultrasound (GF-UC160P-OL5 EVIS EXERA, Olympus America Inc., Melville, NY, USA) when EUS-FNA was indicated, with the Aloka - ProSound Alpha 10 ultrasound system. The procedures were performed by an expert in endoscopic ultrasound (more than 200 procedures/year and over 10 years of experience). The patients were placed in the left lateral recumbent position and received deep sedation prescribed by an anaesthetist. Prophylactic antibiotic therapy was administered to all patients undergoing EUS-FNA. The 22G needle-catheter system was used in most cases, and occasionally 25 and 19G (Boston Scientific Corp, Marlborough, USA) inserted through the working channel of the echoendoscope and advanced into the lesion using real-time ultrasound-guidance. The access was transgastric or transduodenal depending on the location of the lesion. After the removal of the stylet, suction and aspiration was performed with a 10ml syringe to obtain the cyst fluid. In most patients, only a single pass was necessary. In six patients, two passes were required, and in three patients, three passes. All the patients were admitted for 24h after the procedure.
The colour of the cyst fluid (transparent versus non-transparent) and viscosity were examined. It was considered viscous if the length of the mucus chain between the thumb and the index finger of the examiner was ≥1cm for ≥1s (string sign)11 or was clearly string-like.
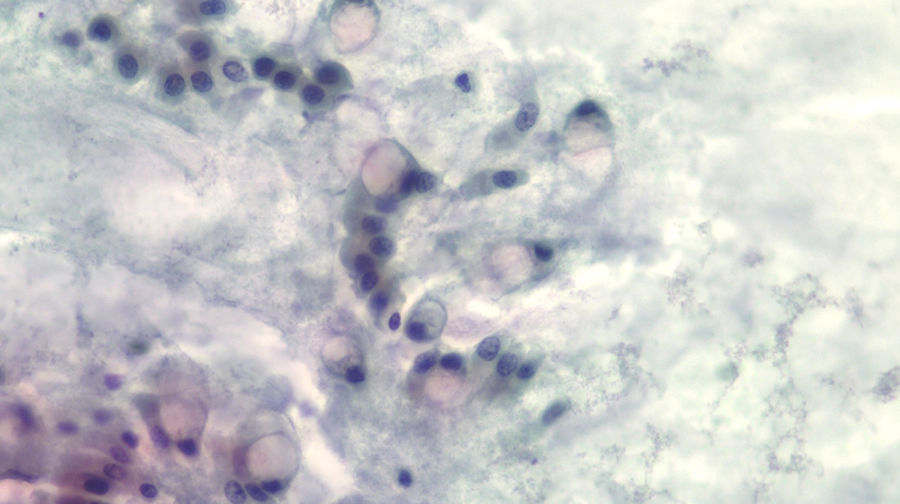
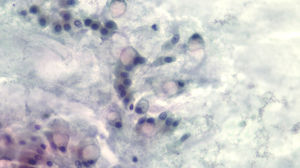
The diagnosis of mucinous cystic lesion by cytology was defined as the presence of: 1) mucinous epithelium: existence of groups of epithelial cells in a single layer, cuboid-shaped (more typical of MCN) or column-shaped (more typical of IPMN), or papillae of mucosecretory cells with intracytoplasmic mucin with or without dysplasia; and/or 2) dense extracellular mucin (Fig. 2).
Part of the aspirated fluid was centrifuged and the supernatant was used to measure for CEA. The quantification of CEA in the cyst fluid was performed using the electrochemiluminescence (ECL) technique (Cobas e 411, Roche, Tokyo, Japan). If the resulting value was greater than 3.8ng/ml, direct dilution of the sample was subsequently carried out.
The PCL were classified as mucinous or non-mucinous cystic lesions. MCN and IPMN were included in the mucinous cystic lesion group. Non-mucinous cystic lesions included: serous cystic neoplasm (SCN), pseudocyst, cyst associated with chronic pancreatitis and other lesions, which sometimes can have a cyst-like appearance, such as neuroendocrine tumours (NET) and ductal adenocarcinoma.
The study included: 1) patients with a confirmed diagnosis of PCL from histology of the surgical specimen; 2) clinical confirmation of pseudocyst and/or 3) image compatible with SCN. Pseudocyst was considered when the patient had a history of acute pancreatitis, cytology showing inflammatory cells and absence of neoplastic cells, papillary epithelium and/or mucin, and disappearance of the cyst with no recurrence over at least one year of follow-up, or significant decrease in cyst size (50%) detected on CT and MRI images assessed by an expert radiologist. SCN was considered when the lesion had a typical honeycomb pattern in the endoscopic ultrasound, non-mucinous cytology (absence of mucin and papillary epithelium) and was stable for at least one year in the follow-up imaging tests.
Statistical analysisThe qualitative variables are expressed in absolute values and their percentages, while the continuous variables are expressed as mean±standard deviation. The proportions were compared with the Chi-square test, while the quantitative exposure variables with categorical response variables were compared with the logistic regression model (2 categories) or multinomial logistic regression (>2 categories). The optimal cut-off point of the CEA quantitative variable was determined by a receiver operating characteristic (ROC) curve to predict mucinous PCL. The area under the ROC curve (interpretation of clinical utility in terms of predictive probability) was calculated. The cut-off point selected was the one that maximised the proportion of correctly classified PCL. The sensitivity, specificity and diagnostic accuracy of cytology, the viscosity of the cyst fluid, the CEA and the combination of the three tests were also calculated. Data analysis was performed with the Stata® statistical package version 12. Statistical significance was considered if the p-value was less than 0.05.
ResultsGeneral characteristics of the patients and pancreatic cystic lesionsA total of 122 patients were referred for EUS. EUS-FNA was performed in 94 patients and puncture was ruled out in 28 for the following reasons: small PCL (less than 10mm) (n=22); access difficulty (n=3); interposition of vessels (n=2); and typical serous endoscopic appearance (n=1). In total, 21/94 patients were operated on with the following histology: four IPMN without dysplasia; two IPMN with dysplasia, six MCN; one cystadenocarcinoma; four SCN; two NET; one chronic pancreatitis; and one ductal adenocarcinoma. The remaining 73 patients who did not have surgery were monitored to check their progress. Of these, seven were clinically defined as pseudocysts and five as SCN by imaging. Ultimately, 33 patients (13 mucinous and 20 non-mucinous PCL) were included in the study.
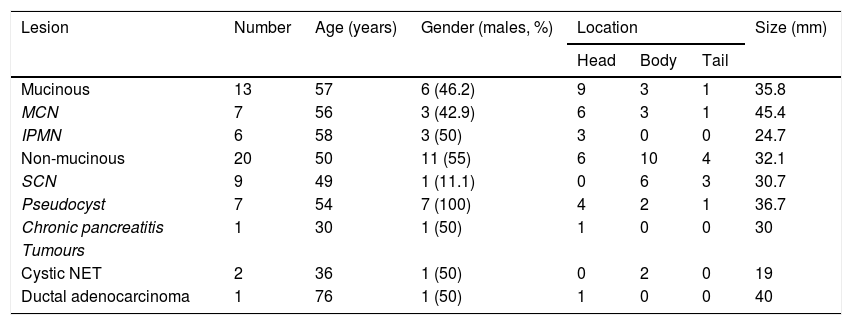
A summary of the general characteristics of the patients is shown in Table 1. Seventeen patients (51.5%) were male and there was a mean age of 53±17.6 years (range: 19–83). The most common location was head of the pancreas (n=15; 45.4%), followed by body (n=13; 39.4%) and tail (n=5; 15.2%). The mean size of the PCL was 33.6±16mm (range: 13-70). Age, gender, location and size of the cyst were similar in both study groups. However, males predominated in all non-mucinous PCL except SCN. The mean age was around 50 years, except in patients with chronic pancreatitis, cystic NET and ductal adenocarcinoma. The most common location was in the head of the pancreas, except in SCN and cystic NET, which were more common in the body. Cystic NET followed by IPMN had the smallest mean size, at less than 3cm. In the study group, 6/33 malignant lesions (18.2%) were detected: two malignant IPMN; one cystadenocarcinoma; one infiltrating ductal adenocarcinoma; and two cystic NET.
Baseline characteristics of the patients.
| Lesion | Number | Age (years) | Gender (males, %) | Location | Size (mm) | ||
|---|---|---|---|---|---|---|---|
| Head | Body | Tail | |||||
| Mucinous | 13 | 57 | 6 (46.2) | 9 | 3 | 1 | 35.8 |
| MCN | 7 | 56 | 3 (42.9) | 6 | 3 | 1 | 45.4 |
| IPMN | 6 | 58 | 3 (50) | 3 | 0 | 0 | 24.7 |
| Non-mucinous | 20 | 50 | 11 (55) | 6 | 10 | 4 | 32.1 |
| SCN | 9 | 49 | 1 (11.1) | 0 | 6 | 3 | 30.7 |
| Pseudocyst | 7 | 54 | 7 (100) | 4 | 2 | 1 | 36.7 |
| Chronic pancreatitis | 1 | 30 | 1 (50) | 1 | 0 | 0 | 30 |
| Tumours | |||||||
| Cystic NET | 2 | 36 | 1 (50) | 0 | 2 | 0 | 19 |
| Ductal adenocarcinoma | 1 | 76 | 1 (50) | 1 | 0 | 0 | 40 |
Only one patient (1/33, 3%) was on antiplatelet therapy (acetylsalicylic acid 100mg/day) and none was on anticoagulants. No serious complications were recorded. Only two patients had self-limiting intra-cyst bleeding after the puncture, but no prolongation of hospital stay or any other additional measures were necessary; neither of them were the patient on antiplatelet therapy.
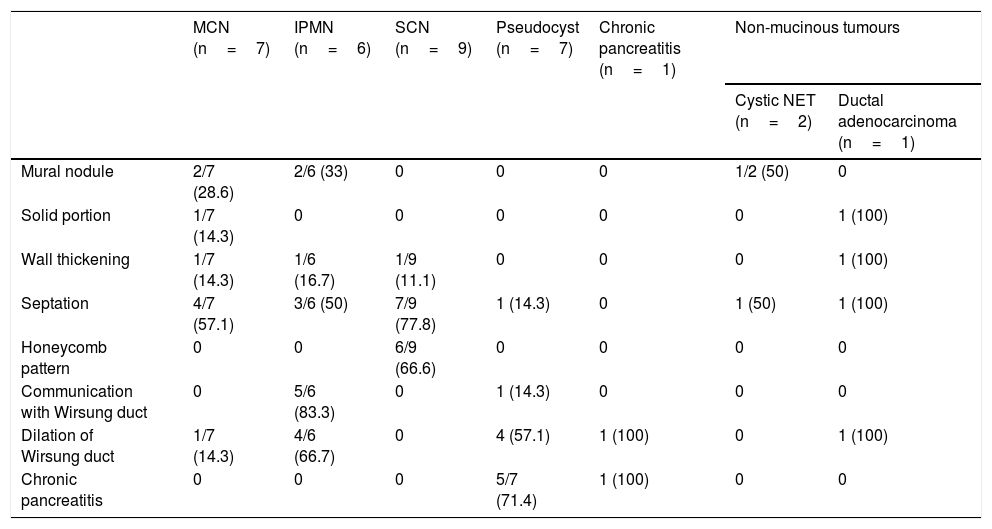
Morphological results of the endoscopic ultrasoundThe ultrasound characteristics of the PCL are described in Table 2. The majority of mural nodules were detected in the mucinous PCL group (4/5), and none was in non-tumour or serous lesions. There was only one solid portion in malignant lesions (ductal adenocarcinoma and cystadenocarcinoma). However, septation was identified in both study groups.
Ultrasound characteristics of the pancreatic cystic lesions.
| MCN (n=7) | IPMN (n=6) | SCN (n=9) | Pseudocyst (n=7) | Chronic pancreatitis (n=1) | Non-mucinous tumours | ||
|---|---|---|---|---|---|---|---|
| Cystic NET (n=2) | Ductal adenocarcinoma (n=1) | ||||||
| Mural nodule | 2/7 (28.6) | 2/6 (33) | 0 | 0 | 0 | 1/2 (50) | 0 |
| Solid portion | 1/7 (14.3) | 0 | 0 | 0 | 0 | 0 | 1 (100) |
| Wall thickening | 1/7 (14.3) | 1/6 (16.7) | 1/9 (11.1) | 0 | 0 | 0 | 1 (100) |
| Septation | 4/7 (57.1) | 3/6 (50) | 7/9 (77.8) | 1 (14.3) | 0 | 1 (50) | 1 (100) |
| Honeycomb pattern | 0 | 0 | 6/9 (66.6) | 0 | 0 | 0 | 0 |
| Communication with Wirsung duct | 0 | 5/6 (83.3) | 0 | 1 (14.3) | 0 | 0 | 0 |
| Dilation of Wirsung duct | 1/7 (14.3) | 4/6 (66.7) | 0 | 4 (57.1) | 1 (100) | 0 | 1 (100) |
| Chronic pancreatitis | 0 | 0 | 0 | 5/7 (71.4) | 1 (100) | 0 | 0 |
IPMN: intraductal papillary mucinous neoplasm; MCN: mucinous cystic neoplasm; NET: neuroendocrine tumour; SCN: serous cystic neoplasm.
Data presented as number (%).
The macroscopic appearance of the fluid (colour and viscosity) was examined in all cases included except one (n=32), where very little fluid was obtained and there was only enough for the cytology smear.
In terms of the colour of the cyst fluid obtained, 22 were transparent and 10 non-transparent. The comparison assessment was ruled out in 3/10 because it was blood-stained, probably secondary to puncture trauma. There was no significant correlation between the transparency (22/29) of the cyst fluid and mucinous lesions (p=0.10).
Viscosity was found in 7/32 PCL punctures, and all seven were mucinous lesions (p<0.01). The specificity was therefore 100%. The data relating to the sensitivity and diagnostic accuracy of viscosity for the determination of mucinous PCL are shown in Table 3.
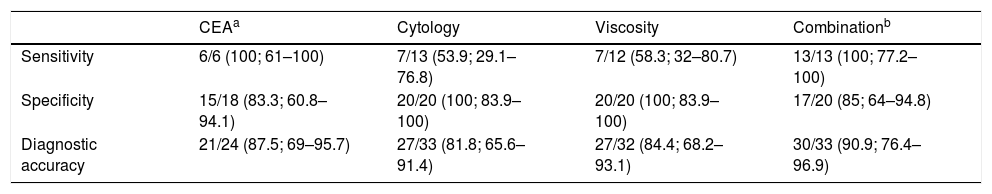
Sensitivity, specificity and accuracy of cytology, CEA in cystic fluid, viscosity and combination of the three in the diagnosis of mucinous pancreatic cystic lesions.
| CEAa | Cytology | Viscosity | Combinationb | |
|---|---|---|---|---|
| Sensitivity | 6/6 (100; 61–100) | 7/13 (53.9; 29.1–76.8) | 7/12 (58.3; 32–80.7) | 13/13 (100; 77.2–100) |
| Specificity | 15/18 (83.3; 60.8–94.1) | 20/20 (100; 83.9–100) | 20/20 (100; 83.9–100) | 17/20 (85; 64–94.8) |
| Diagnostic accuracy | 21/24 (87.5; 69–95.7) | 27/33 (81.8; 65.6–91.4) | 27/32 (84.4; 68.2–93.1) | 30/33 (90.9; 76.4–96.9) |
CEA: carcinoembryonic antigen.
Data are presented as number (%; 95% confidence interval).
In 9/33 cases, the CEA level could not be determined due to the small amount of cyst fluid obtained (approximately 0.5ml) and prioritising the cytology study, or because of its viscosity. The final diagnoses in these nine patients were: mucinous tumours in seven cases (one cystadenocarcinoma, five IPMN and one MCN); and two non-mucinous tumours (one SCN and one pseudocyst). Cytology was diagnostic in 8/9 patients (non-diagnostic in SCN) and the fluid obtained was viscous in all mucinous lesions.
The median concentration of CEA in cyst fluid was higher in mucinous lesions than in non-mucinous lesions: 3,044ng/ml (interquartile range: 1073–5941.5; total range: 419–13,407) versus 7ng/ml (interquartile range: 0.58–168.5; total range: 0–4167) (p=0.02). There were no statistically significant differences in CEA levels in the malignant mucinous lesions compared to the rest (pre-malignant) (p=0.80). CEA levels were <5ng/ml in nine PCL, all of them non-mucinous: six SCN, two NET and one pseudocyst.
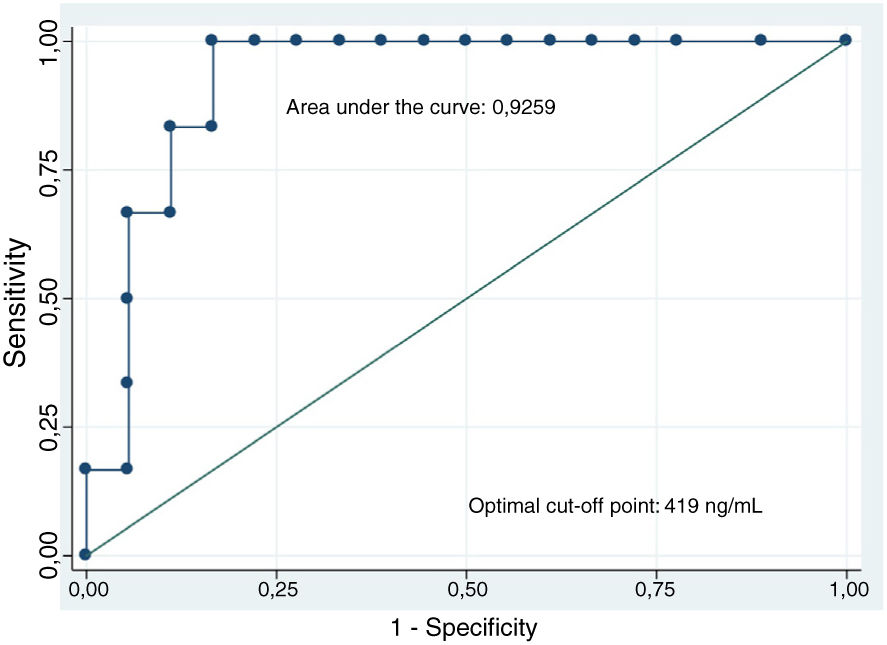
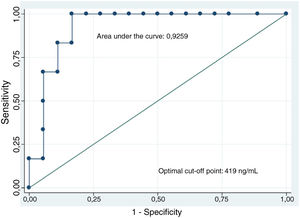
The analysis of CEA levels by means of a ROC curve selected the value ≥419ng/ml as the optimal cut-off point for differentiating mucinous from non-mucinous cystic lesions, with an area under the curve of 0.9259 (95% confidence interval [CI]: 0.7–0.99) (Fig. 3). With this cut-off point, the sensitivity, specificity and diagnostic accuracy were 100%, 83.3% and 87.5%, respectively (Table 3). When using the cut-off point ≥192ng/ml described in the largest series of patients published, the sensitivity does not vary (100%). However, the specificity and diagnostic accuracy are slightly lower (77.8% and 83.3%, respectively).8
CytologyThe cytology results were also categorised into mucinous and non-mucinous PCL. Table 3 shows the sensitivity and diagnostic accuracy results, which were the lowest of all the tests. The specificity was 100%, consistent with the viscosity specificity, and they are both superior to the CEA determination.
Combination of testsThe combination of the three tests (CEA in cyst fluid, cytology and viscosity) was analysed to predict mucinous/non-mucinous PCL. In this combination, PCL were considered mucinous if any of the components of the tests was positive.11 The sensitivity was as high as that of the CEA (100%), with a specificity slightly higher than with CEA (85% versus 83.3%). The diagnostic accuracy of CEA (87.5%) was slightly higher than cytology (81.8%) and viscosity (84.4%). Lastly, the overall diagnostic accuracy of the combination was superior to the three individual tests (90.9%) (Table 3).
DiscussionThese days, it is common to diagnose a PCL in an asymptomatic patient by means of an imaging test.12 The importance of the differential diagnosis of these lesions is that the mucinous type are malignant or potentially malignant and their definitive treatment (surgery) is aggressive. However, most non-mucinous (serous) lesions do not require treatment unless they are symptomatic.13,14
The diagnostic accuracy of imaging techniques (CT, MRI) is poor for differentiating between mucinous and non-mucinous cystic lesions.15 In contrast, EUS provides us with detailed information about the PCL and the rest of the pancreas. However, in many cases it is not possible to establish the diagnosis from the morphological characteristics. In a multicentre study with 112 patients by Brugge et al., the diagnostic accuracy rate was 51%.8 According to the clinical practice guidelines, the size of the PCL (≥3cm) and the presence of mural nodules and/or solid component are risk factors for malignancy.13,14 In our series, mural nodules were only detected in malignant or pre-malignant lesions (two MCN, two IPMN and one NET) and solid portion in malignant lesions (ductal adenocarcinoma and cystadenocarcinoma). However, septation was identified in both study groups.
The advantage of endoscopic ultrasound over other imaging techniques is being able to perform fine-needle aspiration and analysis of the fluid inside the cyst. In clinical practice, cytology and viscosity, biochemistry and tumour marker tests are generally carried out. The macroscopic appearance of the fluid can be very helpful, as mucinous cysts usually have clear and very viscous fluid. Viscosity information can be obtained quickly and easily with high specificity for the diagnosis of mucinous lesion using the previously described string sign. In a series of 98 patients, the specificity was 95%, which was similar to our results (100%). However, it should be taken into account that the sensitivity was 58%, also similar to ours (58.3%).11
Cytology has high specificity (above 90%) but, unlike with solid pancreatic lesions, it has poor sensitivity (around 50%) in PCL.16 The reason is the low number of cells in the cyst fluid aspirate. Similarly, in our study, the sensitivity of cytology was 54% with a specificity of 82%.
The only marker that is currently routinely tested for in the study of PCL is CEA. This was defined following a prospective, multicentre study that evaluated the combination of morphological data, cytology and tumour markers (CEA, CA 19-9, CA 72-4, CA 125 and CA 15-3).8 In our study, the analysis of CEA levels by means of a ROC curve selected the value ≥419ng/ml as the optimal cut-off point to differentiate between mucinous and non-mucinous cystic lesions. This figure is within the range described in previous publications (30−480ng/ml) for the diagnosis of mucinous PCL and its wide variability may be due to the heterogeneity of the studies, as well as the absence of a validated method for processing the sample (to dilute or not, addition of fixative, etc).17,18 According to the literature, a CEA<5ng/ml is predictive of non-mucinous PCL in 94% of cases.19 This is in line with our results, where none of the patients with CEA levels below 5ng/ml had mucinous lesions. Additionally, it has been reported that the increase in CEA does not correlate with the malignancy of the PCL.20 In our study there were also no statistically significant differences in CEA levels to distinguish between malignant and non-malignant lesions (p=0.80).
It was not possible to measure the CEA level in 27.7% of the fine-needle aspirations due to the smallness of the PCL, meaning either that there was very little fluid or it was very dense. Other authors confirm these findings7 and reaffirm the recommendations of the international guidelines for performing fine-needle aspirations only on PCL of at least 2cm.13,14 However, it is possible that lesions smaller than 2cm may be tumours. In our results, two of the lesions smaller than 2cm were tumours (a NET and an IPMN), so caution is required when applying clinical practice guidelines and a personalised approach should be taken. If the lesion measures more than 1cm and the patient is young or has some morphological data of concern (e.g., nodules), a FNA should probably be performed for cytology, even if CEA cannot be determined.
The combination of several tests provides better accuracy in the diagnosis of mucinous PCL, as shown in the literature.8,21 Oppong et al. obtained sensitivity, specificity and diagnostic accuracy of 91%, 75% and 86%, respectively, by combining morphology, cytology and CEA in a series of 133 patients.21 Bick et al. designed a sequential interpretation model that included the analysis of cytology, mucin staining, CEA and string sign, obtaining sensitivity, specificity and diagnostic accuracy of 88%, 92% and 89%, respectively.11 Similarly, the overall diagnostic accuracy in our study combining cytology, string sign and CEA was 90.9%, higher than the three individual tests.
As far as limitations are concerned: 1) this was a single-centre study in a tertiary hospital in Spain; 2) all procedures were performed by an expert in endoscopic ultrasound with access to previous imaging studies, which could have influenced the results of the endoscopic ultrasound; 3) the sample size was small, although it was the largest series published here in Spain; and 4) non-mucinous lesions are included without histological diagnosis, but which meet diagnostic clinical and/or radiological criteria, similar to that previously published.8,9,21
In an attempt to improve EUS-FNA results, new diagnostic tools are being applied to increase its accuracy. The application of accessory techniques in EUS such as contrast,22,23 new instruments for obtaining the sample such as biopsy micro-forceps,24 other complex endoscopic procedures such as the one based on confocal laser endomicroscopy25 and various advanced molecular studies such as the determination of GNAS, KRAS or miRNA26–28 may in the future help with the diagnosis of PCL. However, unlike the tests used in our study, many of these techniques are not available in routine clinical practice.
In conclusion, we assessed the utility of EUS-FNA in PCL by studying the macroscopic characteristics of the cyst fluid, CEA and cytology, as well as the combination of the three parameters, to differentiate between mucinous and non-mucinous lesions. We selected the CEA value ≥419ng/ml as optimal cut-off point for this differentiation. However, it may not be possible to extrapolate this to other populations and it might be necessary for each centre to make their own calculation. The techniques used are simple and accessible in routine clinical practice, obtaining a high degree of diagnostic accuracy (91%) when used in combination, similar to the results of previous studies conducted outside Spain. It would therefore seem that these results can be extrapolated to other populations.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Argüello L, Sánchez-Montes C, Mansilla-Vivar R, Artés J, Prieto J, Alonso-Lázaro N, et al. Rendimiento diagnóstico de la punción mediante ultrasonografía endoscópica de las lesiones quísticas del páncreas. Gastroenterol Hepatol. 2020;43:1–8.