Anorectal disease is a common finding in men who have sex with men (MSM). The most common causes tend to be HIV-related or non-HIV-related infections. These causes include lesions caused by herpes simplex virus, Treponema pallidum, Neisseria gonorrhoeae (N. gonorrhoeae), Chlamydia trachomatis (C. trachomatis), Shigella spp. and protozoan infections. Invasive C. trachomatis genotypes (L1-L3) cause lymphogranuloma venereum (LGV), which used to be considered a rare finding in developed countries.1 However, since 2003, numerous cases have been reported in Europe and the United States, primarily among MSM, with the main infections manifested including proctitis2 associated with circulation of the L2b variant. Diagnosis has improved thanks to the use of molecular techniques on direct samples, and genotyping of LGV-producing genovars. Treatment for LGV is not completely standardised since doxycycline or azithromycin can be used with variable doses and duration of therapy. This paper looks at an uncommon cause of proctitis due to co-infection by C. trachomatis and Haemophilus parainfluenzae (H. parainfluenzae).
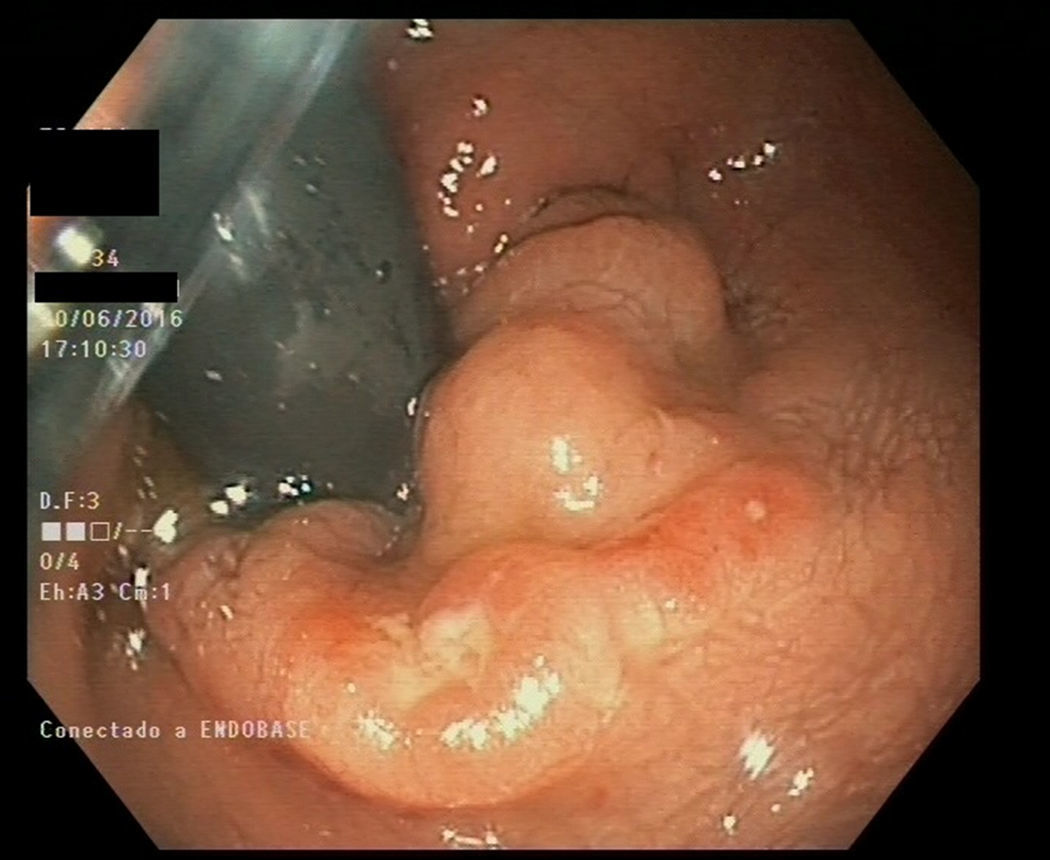
The patient is a 34-year-old male who has unprotected sex with other men. The patient has a history of acute hepatitis B from 2013 and has now achieved seroconversion (HBsAg negative, HBsAb positive) with secondary syphilis. He is not infected with HIV. He is a regular user of cocaine, inhaled nitrates, speed and lysergic acid derivatives. He has a cat, a boa constrictor and 12 rats, which do not get veterinary health check-ups. The patient attended the emergency department after suffering from severe proctalgia for 2 days, accompanied by constipation, self-limited episode of rectal bleeding and a fever of 38°C with no apparent source. One week ago, he manually extracted stool from his rectum (self-disimpaction). A non-specific rectal ulcer with raised edges was observed on the colonoscopy with central depression and fibrinous base (Fig. 1). Biopsies were taken for examination. The patient was polymerase chain reaction (PCR) positive for LGV-related genotypes in laboratory tests. Subsequent allele-specific PCR based on 3 aa (9 pb) deletion between L2 positions 591–592 and pmpH gene sequencing determined that the strain belongs to the invasive L2 genotype3 as it showed the specific P159L mutation. Growth of H. parainfluenzae was observed in culture media. An E-test was performed to test antimicrobial susceptibility; it was susceptible to trimethoprim/sulfamethoxazole (MIC: 0.19mg/l), ampicillin (MIC: 0.38mg/l), amoxicillin-clavulanic acid (MIC: 0.38mg/l), cefixime (MIC: <0.016mg/l), levofloxacin (MIC: 0.064mg/l), tetracycline (MIC: 0.047mg/l) and azithromycin (MIC: 3mg/l). Finally, doxycycline 100mg/12h was prescribed for 21 days. The patient has been asymptomatic since then.
No previous case of rectal ulcer with the presence of H. parainfluenzae, either alone or with other microorganisms, has been described to date. Finding both H. parainfluenzae and LGV genotype L2, which is less prevalent in our environment than genotype L2b, primarily associated with the re-emergence of LGV in Europe, makes this a noteworthy, previously unpublished case, which coincides with that indicated for LGV in European papers,4 but is different from that detected in nearby geographical areas.5 The patient's symptoms may of course be due almost exclusively to LGV infection and not to Haemophilus, although this can behave as an opportunistic pathogen and take advantage of the epithelial lesion caused by LGV.
We are currently witnessing epidemiological changes in the aetiology of sexually transmitted infections and/or infections in unusual sites, which will require a more complete diagnostic approach, with the requirement for more testing and adaptation of available diagnostic procedures to clinical needs.6–8 In cases of proctitis, especially in MSM, use of anoscopy or rectosigmoidoscopy with sampling should be assessed, regardless of whether there is a lesion or not. However, there are currently no parallel cost-effectiveness studies showing whether such use is profitable in our health system. It must be remembered that there are no pathognomonic lesions for this type of disease, and lesions can sometimes be confused with those that are secondary to inflammatory bowel disease. Microbiological culture of fastidious bacteria, PCR assay for the detection of herpes simplex virus, N. gonorrhoeae and C. trachomatis, and their serotyping, when possible, are considered essential tests.
C. trachomatis infection, and specifically the onset of LGV in developed countries, has been shown to be linked to HIV infection (in up to 70% of cases) in MSM with multiple partners, but no link has been shown with the level of immunosuppression. In our case, the patient was not infected with HIV. Other independent risk factors include a history of sexually transmitted diseases, unprotected anal intercourse, travel abroad and encounters with sexual partners found via the internet.9,10
Please cite this article as: Caballero-Mateos AM, López de Hierro-Ruiz M, Rodríguez-Domínguez M, Galán-Montemayor JC, Gutiérrez-Fernández J. Co-infección por linfogranuloma venéreo y Haemophilus parainfluenzae durante un episodio de proctitis. Gastroenterol Hepatol. 2018;41:107–109.