The introduction of treatment with tumour necrosis factor inhibitors (anti-TNF) has brought about a change in the management of patients with inflammatory bowel disease (IBD) who do not respond adequately to conventional therapies with steroids and immunomodulators. Several biological treatments with this mechanism of action and proven efficacy in both the induction and maintenance of clinical remission are currently available.1 For Crohn's disease (CD), infliximab, adalimumab and certolizumab pegol are available (the latter only in the USA and Switzerland2,3). A recent meta-analysis4 comparing these therapies found no differences between them. The use of infliximab, adalimumab and, more recently, golimumab, has been approved in the case of patients with ulcerative colitis (UC).5–7 Although the therapeutic panorama has expanded with the emergence of these drugs, with high rates of response and remission, around 20–40% of patients with CD and 30–40% with UC do not respond to treatment (primary failure).8 Another group of patients who respond initially but subsequently lose the response, known as secondary non-responders, are not clearly defined in the different studies. In most cases, response is lost during the first year of treatment at a rate of between 23% and 46% at 12 months in patients with CD on infliximab or adalimumab treatment, if secondary loss of response is defined as the need for dose escalation.9 The annual loss of response per patient is estimated be around 13% with infliximab10 and 20% with adalimumab11 in CD. In UC, one study found a secondary loss of response of approximately 59% during infliximab and adalimumab treatment, taking into account both treatment escalation as well as surgery or the requirement for rescue treatment with steroids.12 When there is loss of response, the anti-TNF dose is generally up-titrated, changed to another anti-TNF, or switched to another drug with a different mechanism of action. Several studies have suggested that measuring anti-TNF antibodies as well as drug levels could benefit patient management in these cases, thereby optimising the use of these treatments. This review aims to analyse the current literature to evaluate the value of applying these measurements in clinical practice.
Pharmacokinetics of tumour necrosis factor inhibitorsLack or loss of response can be affected by inter- and intra-individual variability in drug bioavailability and pharmacokinetics, which depends on the structure of the drug, route of administration, degradation and elimination. Infliximab is given intravenously, allowing large volumes of drug to be administered to reach a peak concentration almost immediately after the infusion, obtaining rapid distribution with low inter-individual variability. Adalimumab, certolizumab and golimumab are administered subcutaneously, with a lower drug volume and slower absorption via lymphatic drainage. These drugs reach peak concentrations 5–10 days later and have a bioavailability of 50–100%, leading to greater inter-individual variability.13,14 The mechanism of degradation and elimination of anti-TNFs is not precisely known, although it is believed to occur mainly by proteolytic catabolism in the reticuloendothelial system after antibody receptor-mediated endocytosis.15 There are neonatal Fc receptor-mediated mechanisms that protect against catabolism, recycling the IgG antibodies and returning them to the circulation, thus increasing their half-life.16,17 This receptor has a greater affinity for human IgG, the half-life of which varies according to the level of humanisation of the antibody, so that murine antibodies have a half-life of 1–2 days, chimeric antibodies 10–14 days, and humanised antibodies 10–20 days.16,18 Nevertheless, these mechanisms can become saturated in certain situations, such as systemic inflammatory diseases, decreasing the half-life of the drug.13–16 Another clearance mechanism involves modification of the drug structure, as in the case of certolizumab, in which the Fc portion is replaced by polyethylene glycol, increasing its half-life by decreasing its proteolysis and immunogenicity.19
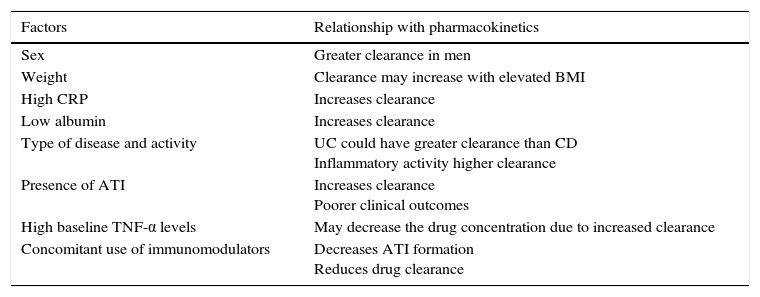
There are other factors that affect drug clearance, such as albumin and C-reactive protein (CRP) levels, body mass index, sex, type of disease (CD or UC) and activity, inflammatory cytokines, concomitant use of immunomodulators and development of anti-drug antibodies (ADA).17,20 One study that evaluated these factors in 169 patients with CD and UC observed that low albumin levels, high CRP levels and the presence of antibodies to infliximab (ATI) were significantly associated with increased infliximab clearance.21 Several studies associating these factors with the pharmacokinetics of biological drugs are summarised in Table 1.20,22
Factors related with the pharmacokinetics of the anti-TNF drugs.
| Factors | Relationship with pharmacokinetics |
|---|---|
| Sex | Greater clearance in men |
| Weight | Clearance may increase with elevated BMI |
| High CRP | Increases clearance |
| Low albumin | Increases clearance |
| Type of disease and activity | UC could have greater clearance than CD Inflammatory activity higher clearance |
| Presence of ATI | Increases clearance Poorer clinical outcomes |
| High baseline TNF-α levels | May decrease the drug concentration due to increased clearance |
| Concomitant use of immunomodulators | Decreases ATI formation Reduces drug clearance |
Anti-TNF drugs are IgG1 monoclonal antibodies. The immune system may not recognise biologic treatments as its own, generating ADAs, which increase drug clearance due to the formation of immune complexes that are eliminated by the reticuloendothelial system.
Antibodies are directed against the F(ab)2 variable region, which is better characterised in the case of infliximab,23 but ADA formation has also been described in the case of adalimumab and certolizumab.
Various factors affect the development of ADA, including biological structure, the immune status of the patient, the route of administration, the treatment regimen (maintenance or episodic) and concomitant medication.24 Due to this and the fact that ADA are measured using different techniques and at different times, their incidence varies greatly in different studies. ATI formation ranges from 6% to 61%.25,26 The ACCENT I study in patients with CD describes ATI formation in between 6% and 28% of patients, taking into account that the results were inconclusive in 46% of patients due to the detection of serum infliximab that interfered with the determination of ATI by enzyme-linked immunosorbent assay (ELISA).25 In the case of the antibodies to adalimumab (ATA), 2.6% of patients in the CLASSIC II study on adalimumab maintenance therapy in CD had developed ATA at week 56.27 However, in studies in rheumatological diseases, especially rheumatoid arthritis, ATA levels of between 12% and 17% were described.28,29 In the PRECISE studies, antibodies against certolizumab appeared in 8–9% of patients at week 26.30,31 Finally, 2.9% of patients in the PURSUIT study on maintenance therapy with golimumab in UC developed ATA.7
As previously mentioned, maintenance therapy results in a lower incidence of ADA compared to episodic treatment.26,32–34 In a study conducted by Hanauer et al.,33 antibody formation was significantly higher in patients on episodic treatment (30%), while incidence in the maintenance groups was 7% and 10%. Levels were also lower in patients taking combined immunosuppressive therapy. In a meta-analysis of 3326 patients with CD and UC, Lee et al.34 found the total prevalence of ATI to be 20.8%: 45.8% in episodic infusion, and 12.4% in maintenance treatment (p<0.00001).
It is important to take into account that the appearance of ADAs may be transient, and that they do not always have a clinical impact. This was shown in a study by Vande Casteele et al.,35 in which the authors analysed 1232 consecutive serum samples from 90 patients with CD and UC treated with infliximab. Of the patients who presented ATI, 28% were transient and 72% persistent, observing that patients in the group with persistent ATI had higher mean ATI levels with respect to the transient group, with a greater need to discontinue treatment (68% vs 13%; p=0.0005). Exactly why these antibodies disappear is unclear, although this study reports that 20% of patients received concomitant immunosuppressive treatment, dose escalation was required in 20%, and the cause was unknown in the remaining 60%. In another study by Steenholdt et al.,36 antibodies were observed to disappear in two-thirds of patients who presented a clinical response and to persist in non-responders. When infliximab was discontinued, the antibody levels decreased over time, but were able to persist for several years.
Most patients who develop persistent ATI do so within the first 12 months of treatment, while transient ATI can appear at any time during treatment. In some cases, onset of clinical loss of response can be preceded by the development of ATI.37
Another factor implicated in the formation of ADA is concomitant therapy with immunomodulators, which is associated with less antibody formation,26,38–40 with the risk of developing ATI decreasing by up to 50% in some studies.34 Vermeire et al.,38 in a cohort of 174 patients with CE, evaluated the appearance of ATI according to the presence of immunosuppressive therapy. They found that patients on concomitant therapy (azathioprine or methotrexate) had a lower incidence of ATI compared to patients without immunosuppression, 46% and 73%, respectively (p<0.001), with no differences between azathioprine and methotrexate. In the SONIC study,39 508 patients with CD were randomised to azathioprine, infliximab or both. At week 30 of follow-up, ATIs were observed in 0.9% of patients on combined therapy compared to 14% in the infliximab group, with fewer infusion reactions in the immunomodulator group compared to the infliximab group (5% and 16.6%, respectively). In another recent study conducted by van Schaik et al.,40 the incidence of ATI was also higher in the monotherapy group compared to the group receiving combination therapy with immunomodulators (29.8% vs 5.7%; p=0.001). However, concomitant administration of immunomodulators should be individualised, as there is a risk that deep immunosuppression can increase side effects.
Measurement methodsTechniques for measuring anti-TNF and ADA levels are very diverse, and include enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, mobility shift assay, reporter gene assay and enzyme immunoassay, the latter used to detect ADA. The most widely used in studies, due to its simplicity, is solid-phase ELISA, a technique that is both sensitive and inexpensive. However, the interaction of infliximab with different immunoglobulins (for example, the assay can recognise the Fc fraction of rheumatoid factor IgG) can cause false positives, and false negatives can arise due to failure to detect antibodies caused by drug interference or the presence of IgG4.18,41 Radioimmunoassay is used fundamentally in the liquid phase, and is more sensitive compared to ELISA. Moreover, it does not interact with other immunoglobulins and detects IgG4; however, it is more complex owing to the use of radioisotopes.42
The mobility shift assay has been validated more recently for the determination of both drug and ADA levels. It has high sensitivity and specificity, can detect all immunoglobulin subtypes, and allows antibodies to be identified despite drug interference, with even lower detection levels compared to previous techniques.43,44
The use of these techniques varies greatly between groups. Several studies comparing different approaches have found good correlation in terms of determination of infliximab concentration and ATI levels, with similar clinical results.45,46 However, differences have been observed in infliximab concentrations and ATI levels, and the same technique should be used in the same patient.47
Clinical impact of drug concentration and anti-drug antibody levelsAnti-drug antibody levelsSeveral studies have observed that the presence of ADAs is correlated with a lower drug concentration, and with an increase in infusion reactions.26,34,48–50
The correlation between the presence of antibodies and remission is less clear and more contradictory in different studies. In a prospective cohort study by Warman et al.48 and in a meta-analysis by Lee et al.,34 the presence of ATI was associated with undetectable infliximab trough levels, with no differences in terms of remission. However, a meta-analysis by Nanda et al.49 in 1378 patients with IBD found a greater loss of response in patients who developed ATI, with a relative risk of loss of response of 3.2 (confidence interval 2.0–4.9; p<0.0001).
Tumour necrosis factor inhibitor concentrationSeveral studies have related drug levels with clinical response and remission, such that if these are adequate, the rate of remission in both CD and UC and with different drugs is higher.32,51–53 Other aspects evaluated are endoscopic remission and biochemical markers such as CRP, with an improvement observed in patients with therapeutic drug levels.52,54–56 The heterogeneity of the studies and the different measuring techniques used make it all but impossible to establish optimal reference values for achieving clinical outcomes. The most relevant studies are discussed below.
A post hoc study of data from the ACCENT I study conducted by Cornillie et al.55 evaluated the utility of measuring infliximab trough levels at week 14 to predict sustained response in patients on maintenance treatment, with follow-up for 54 weeks. Trough levels in the post-induction phase were higher in patients with a sustained response with infliximab compared to those with no sustained response. Predictors of sustained response were found to be trough levels ≥3.5μg/mL and a decrease of ≥60% in CRP values at week 14.
Baert et al.26 analysed a consecutive cohort of 125 patients with CD who received episodic treatment with infliximab, determining the drug and ATI levels prior to and 4 weeks after infusion, and evaluating the clinical response, side effects and concomitant use of immunomodulators. The incidence of ATI was 61%, dividing the patients into 2 groups with a cut-off point of 8μg/mL. Levels of >8μg/ml predicted a shorter duration of response with a higher risk of infusion reactions. The infliximab concentration was significantly related with antibody levels before infusion, with a longer lasting response with drug levels >2μg/mL. Moreover, patients with concomitant immunosuppressive therapy had a lower incidence of ATI.
A study by Vande Casteele et al.35 retrospectively analysed 1232 serum samples from 90 patients with CD and UC treated with infliximab, to assess the relationship of ATIs and drug levels with inflammatory markers and clinical evolution. Receiver operating characteristic (ROC) analysis showed that infliximab trough levels of <3μg/mL at week 6 had high sensitivity and specificity for the formation of ATI, being higher when the levels were <2.2μg/mL at week 14. The presence of trough levels <2.2μg/mL at week 14 also significantly predicted discontinuation of infliximab treatment due to persistent loss of response or infusion reactions (sensitivity 82%, specificity 74%). The authors therefore proposed measuring infliximab trough levels at week 14 after induction or in the event of loss of response; if low or undetectable, ATI should be measured. If the ATI are positive and remain over time, a change of therapy should be considered.
Maser et al.32 analysed a consecutive group of 105 patients with CD who received induction treatment with infliximab followed by episodic or maintenance treatment, in order to evaluate ATI formation, drug concentration and clinical significance. Patients with detectable drug levels were observed to have greater clinical remission at 52 weeks compared to those who had undetectable levels (82% vs 6%; p<0.001), lower CRP levels (2.0 vs 11.8μg/L; p<0.001) and a higher incidence of endoscopic improvement (88% vs 33%; p<0.001). Furthermore, ATI formation and infusion reactions were higher in the episodic treatment group. Another study by Imaeda et al.57 confirmed that endoscopic activity is negatively correlated with drug levels and positively correlated with CRP and faecal calprotectin levels.
In a study of 115 patients with UC on maintenance treatment with infliximab, patients with adequate drug levels were observed to have higher rates of remission and endoscopic improvement, with an increased risk of colectomy in patients with undetectable levels.58
In a study of patients with CD on treatment with adalimumab, drug levels were lower in those who stopped treatment at 6 months due to loss of response compared to those who continued on treatment, and the presence of ATA was associated with lower drug levels (p<0.0001).59 Another more recent study analysed adalimumab levels in a population of patients with CD from the CLASSIC I and CLASSIC II studies, confirming the positive relationship between mean adalimumab levels and remission.60
As previously mentioned, drug concentration is related with the presence of concomitant immunosuppressant treatment: patients on combined treatment had increased drug levels compared to those who did not receive combined treatment with immunomodulators.38,40 In a retrospective study of patients who received treatment with infliximab or adalimumab, drug and antibody levels were evaluated according to the previous use of immunomodulators, observing higher infliximab levels in the combination group compared to the monotherapy group (4.6 vs 7.5μg/mL; p=0.04), with no significant differences with adalimumab.40
Applicability of monitoring anti-drug antibody levels and tumour necrosis factor inhibitor concentration in inflammatory bowel disease in clinical practiceA significant percentage of patients can present loss of response to anti-TNF drugs. In clinical practice, this is usually managed by dose escalation or more frequent administration, switching to another anti-TNF if it fails or to a drug with another therapeutic target, all guided by clinical symptoms and biochemical parameters. This strategy is applied in many patients without obtaining any response, and can increase the risk of side effects.
Most patients developing ADA fail to respond after dose escalation. This was observed in a study by Afif et al.61 in 155 patients in whom ADA and drug levels were measured to evaluate whether dose escalation or changing to another anti-TNF was the most effective strategy. Measurement of ADA and drug levels changed patient management in 73% of cases. In the presence of positive ADA, switching to another anti-TNF achieved a better response than dose escalation (17% vs 92%; p<0.004). In patients with drug concentrations below therapeutic levels, better responses were obtained with dose escalation (86% vs 33%; p<0.016).61 Therefore, unnecessary dose escalation–which is also associated with greater infusion reactions–could be avoided in patients with positive ADA. However, the appearance of antibodies is transient in a number of patients.
Another possible application would be to monitor drug levels in order to reach an appropriate concentration, knowing that this is associated with better remission rates. The disadvantage is that, due to the heterogeneity of the studies, neither the cut-off levels nor the best measurement time point have been validated. Several studies have attempted to address this in recent years, including the TAXIT trial by Vande Casteele et al.62,63 This study, carried out in 275 patients with CD and UC in clinical remission, evaluated the utility of individualised infliximab treatment based on monitoring drug levels, which were adjusted to between 3 and 7μg/mL in an optimisation phase, with a subsequent maintenance phase. In the optimisation phase, patients with therapeutic levels were observed to have lower CRP and ATI levels compared to those with sub-therapeutic levels. The results of the maintenance phase showed that patients with symptom-guided therapy had lower therapeutic drug levels and higher ATI levels compared to patients monitored using drug levels, with no differences in terms of clinical remission. The same group has recently carried out a study similar to TAXIT (pending publication) to assess the clinical aspects of infliximab therapy based on clinical evolution or on drug levels.64 This is a randomised study that includes 263 patients with CD and UC in remission on maintenance treatment with infliximab. In the first phase (optimisation), optimum drug levels of between 3 and 7μg/mL (according to the TAXIT regimen) were achieved by increasing or decreasing the dose. Afterwards, in the maintenance phase, patients were randomised to receive infliximab according to their clinical characteristics or drug levels. Although no significant differences in clinical remission were observed at 1 year (66% and 69%, respectively; p=0.686), differences were found in the relapse rate (17% and 7%, respectively; p=0.018). Furthermore, in the optimisation phase, reducing the dose in the group of patients with high drug levels resulted in a 28% saving in the drug cost (p=0.001), with no major differences in total post-maintenance phase cost between the drug-concentration guided group and the clinical symptom-guided group (€20,723 vs €21,023, respectively).
With regard to cost, 2 studies by Steenholdt et al.,65,66 found that individualised treatment based on drug and anti-TNF levels is cost effective. They evaluated patients with infliximab treatment failure who were randomised to escalation or modification according to drug and antibody levels. No differences were observed as regards response or remission, but costs were significantly lower in the algorithm-guided group, with a 31% reduction in the intention-to-treat population ($11,940 vs $17,236; p=0.005). In the follow-up study, the algorithm-guided strategy continued to be cost-effective at 1 year of treatment.66
In most cases, loss of response is usually secondary to the presence of neutralising antibodies or due to low drug levels, but it can also be due to pathways with different inflammatory mechanisms or the absence of IBD-related activity.67 The use of drug and antibody monitoring could be a new strategy for modifying the therapeutic approach.
Because studies published to date have used different techniques and measurement time points, no therapeutic drug monitoring protocol has yet been validated in IBD. However, a number of studies in this respect are currently underway, including the PREDICROHN study to assess different predictors of short- and long-term response in patients with CD, including anti-TNF and ATI levels.
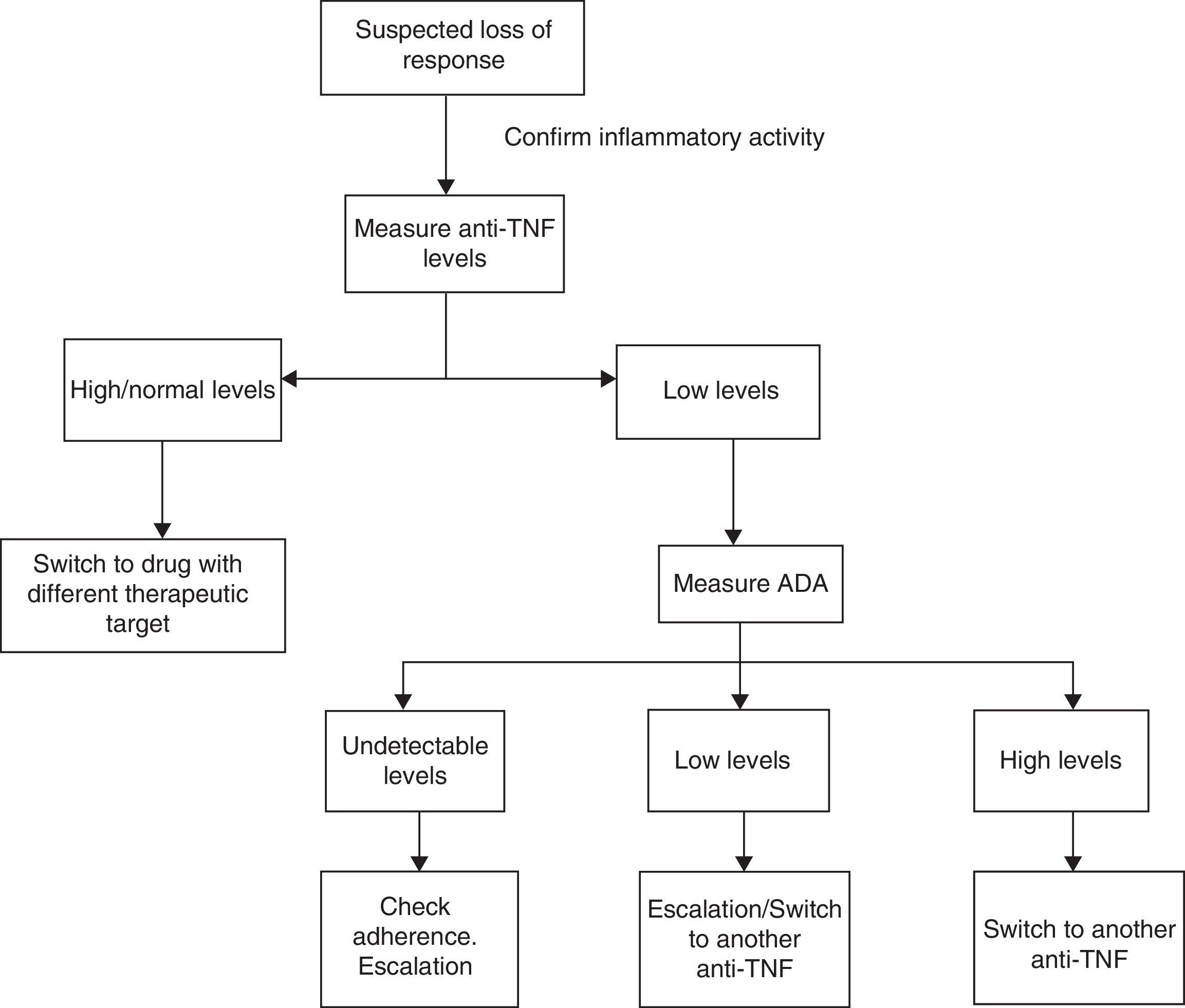
Considering the aforementioned studies, we propose the algorithm shown in Fig. 1. Firstly, when loss of response is suspected, analytical studies with biochemical parameters and endoscopic examinations should performed to confirm that the patient's symptoms are related to IBD and not to other concurrent processes (over-infection, irritable bowel syndrome, stenosis, malabsorption of bile salts, etc.). Once the activity has been confirmed, drug levels should be measured. Based on these, the following situations might be encountered:
- 1.
Supra-therapeutic/normal levels: TNF might not be the inflammatory mediator. Treatment should be changed to another biologic with a different therapeutic target.
- 2.
Sub-therapeutic anti-TNF levels with high ADA levels: most patients do not respond to treatment escalation. The best approach is therefore to switch to another anti-TNF.
- 3.
Sub-therapeutic anti-TNF levels in the absence of ADA: good treatment adherence should first be confirmed. If so, up-titrate the drug.
There are many limitations at present to the widespread use of these algorithms, since studies conducted to date have used different techniques to measure anti-TNF levels and different criteria to define loss of response. The best time point to measure anti-TNF levels has not been clearly established, although some studies propose doing so after induction, at week 14 or when loss of response is suspected. It is also important to remember that in some cases, the presence of ATIs may be transient and not related with clinical deterioration, as mentioned previously, and should therefore be interpreted with caution. Nevertheless, the results are encouraging in terms of optimising resources, avoiding treatment and dose escalation in patients with little likelihood of response.
ConclusionsAnti-TNF drugs have an important role in the management of IBD, and have shown their effectiveness in the induction and maintenance of remission. However, some patients remain unresponsive to these agents. Until now, anti-TNF dose escalation has generally been based on clinical symptoms and biochemical parameters. Monitoring of drug and antibody levels could guide the therapeutic approach in this situation and optimise use of these drugs by reducing costs. This would be particularly beneficial in avoiding unnecessary treatment when drug levels are adequate, since most patients do not respond to dose escalation. Nevertheless, further studies are necessary to validate a therapeutic algorithm that would allow clinicians to optimise resources, extending the study to all anti-TNF drugs and all types of patients with IBD.
Please cite this article as: López-Ibáñez M, Marín-Jiménez I. Niveles de fármaco y anticuerpos antifármaco en el manejo clínico del paciente con enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2016;39:265–272.