Treatment for portal vein thrombosis (PVT) is not well established. Nevertheless, anticoagulation therapy can seemingly be used as first-line therapy. However, there are limited data on the role of this treatment in patients with PVT and cirrhosis. We sought to assess the safety and efficacy of anticoagulation therapy in a series of patients with non-malignant PVT and liver cirrhosis.
MethodsWe analyzed the data of 32 patients with cirrhosis and PVT between March 2009 and September 2015. All patients received anticoagulation treatment. PVT was diagnosed within the context of biannual hepatocellular carcinoma screening in these patients.
ResultsRecanalisation was achieved in 23 patients: complete in 17 patients (53.1%) and partial in 6 patients (18.7%). The median time for achieving a complete response was 7 months (95% CI: 6–8). We did not discover any risk factors associated with repermeation (partial or complete). None of the patients presented with thrombosis progression while receiving anticoagulation. Nine patients who achieved complete recanalisation and stopped anticoagulation therapy suffered rethrombosis (52%). There were no differences between the patients who achieved complete or partial recanalisation (35%) and those who did not (33%) in relation to the onset of hepatic events during follow-up. Three patients (9%) presented with bleeding complications: two variceal bleeding episodes and one brain hemorrhage.
ConclusionsIn cirrhotic patients with non-malignant PVT, anticoagulation therapy led to partial or complete recanalisation in 70% of patients, with a broad safety profile. Due to the existing rethrombosis rate, long-term anticoagulation should be considered.
El tratamiento de la trombosis de la vena porta (TVP) no está bien consolidado. Sin embargo, parece que el tratamiento anticoagulante puede ser el tratamiento inicial. Con todo, hay datos limitados sobre el papel de este tratamiento en pacientes con TVP y cirrosis. Intentamos evaluar la seguridad y eficacia del tratamiento anticoagulante en una serie de pacientes con TVP benigna y cirrosis hepática.
MétodosAnalizamos datos de 32 pacientes con cirrosis y TVP entre marzo de 2009 y septiembre de 2015. Todos los pacientes recibieron tratamiento anticoagulante. La TVP se diagnosticó en el contexto de la detección bianual de carcinoma hepatocelular en estos pacientes.
ResultadosLa recanalización se logró en 23 pacientes: completa en 17 pacientes (53,1%) y parcial en 6 pacientes (18,7%). La mediana de tiempo para conseguir esta respuesta completa fue 7 meses (IC95: 6–8). No descubrimos ningún factor asociado con la posibilidad de reinfiltración (parcial o completa). Ninguno de los pacientes presentó progresión de la trombosis durante la anticoagulación. Nueve pacientes que lograron una recanalización completa y suspendieron el tratamiento anticoagulante presentaron retrombosis (52%). No hubo diferencias entre los pacientes que lograron la recanalización completa o parcial (35%) y los que no lograron la recanalización (33%) en relación con el desarrollo de sucesos hepáticos durante el seguimiento. Tres pacientes (9%) presentaron complicaciones hemorrágicas: dos episodios de sangrado varicoso y una hemorragia cerebral.
ConclusionesEn pacientes cirróticos con TVP benigna, el tratamiento anticoagulante produjo la recanalización parcial o completa en el 70% de los pacientes, con un amplio perfil de seguridad. Debido a la tasa de retrombosis existente, se debe considerar la anticoagulación a largo plazo.
Portal vein thrombosis (PVT) is considered a common complication of liver cirrhosis and the most prevalent thrombotic event in these patients. Its prevalence increases with liver disease severity, reaching 25% in patients awaiting liver transplantation.1–3 In spite of these data, there are no specific recommendations regarding its early detection in cirrhotic patients aside from the screening of hepatocellular carcinoma. Therefore, the most common scenario to diagnose PVT is its asymptomatic detection during routine ultrasound examination. The natural history of PVT in liver cirrhosis is not very well defined,4–6 and it is like that more studies are needed to evaluate whether PVT has a prognostic value in outcomes of cirrhosis.7 In any case, in the context of liver transplantation the deleterious effects of PVT are better known.8,9
There are no established treatment guidelines or a consensus about the optimal treatment for PVT in patients with cirrhosis. However, anticoagulation is considered the first-line therapy in cirrhotic patients, and in fact, several studies report a beneficial effect on recanalization, but the evidence regarding this treatment is based on only a few studies.10–16 A recent meta-analysis comparing anticoagulation treatment vs no therapy in these patients supports this benefit, though limited by the small sample size, too.17 It seems that this therapy in these groups of patients does not carry a high risk of bleeding,18 but for reasons mentioned above firm conclusions cannot be drawn from this.
In this observational study, we address anticoagulation therapy in a group of cirrhotic patients with non-malignant PVT.
Patients and methodsConsecutive patients with cirrhosis and portal vein thrombosis attended to our hospital (Virgen de la Salud, Toledo, Spain) and treated with anticoagulation therapy between March 2009 and September 2015.
PVT was defined as the presence of hyperechoic material within the vein and the absence of flow in part or all the lumen of the portal vein trunk and portal vein branches or portal vein trunk and splenic vein or superior mesenteric vein. Thrombosis was considered partial when thrombus involved less than 50% of the vessel and occlusive when blood flow was absent or the thrombus involved more than 50% of the vessel. PVT was diagnosed with Doppler ultrasound during the six-month scheduled hepatocellular carcinoma screening. A second imaging study, computed tomography (CT) or magnetic resonance imaging (MRI) was done to confirm PVT and to assess its extent.
Anticoagulation therapy was indicated in all patients because of recent thrombosis: when imaging in the previous 6 months demonstrated no thrombosis.
Cirrhosis was diagnosed on the basis of clinical, laboratory and imaging studies.
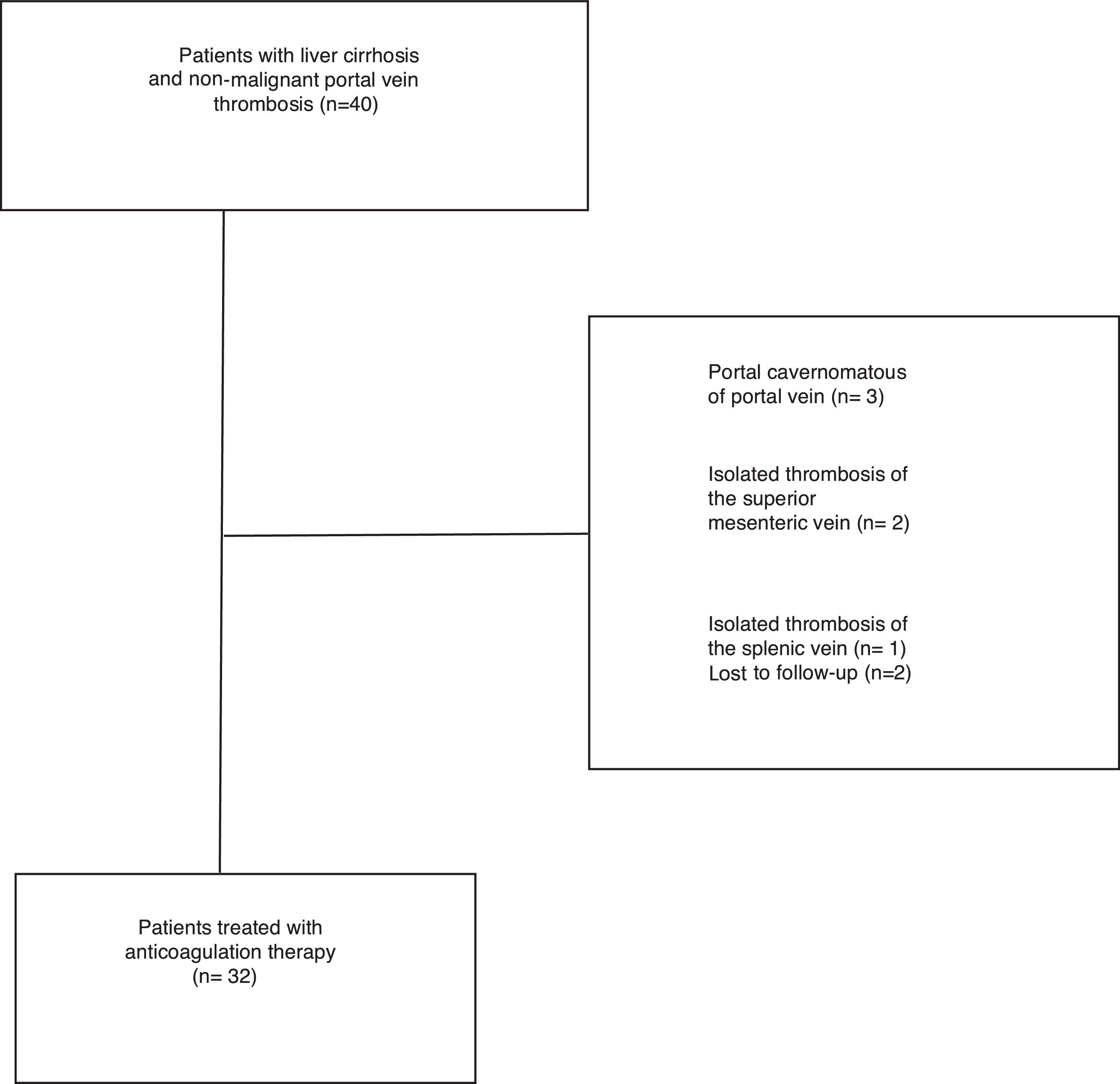
Exclusion criteria included: isolated thrombosis of the superior mesenteric or splenic without involvement of PV, vein cavernomatous transformation of the portal vein and presence of hepatocellular carcinoma or another neoplastic disease (Fig. 1).
Time zero was defined as the date when Doppler ultrasound showed PVT. The time interval until starting anticoagulation was analyzed.
Recanalization was evaluated by Doppler ultrasound or CT/MRI. Patients with complete or partial resolution were regarded as responders. Recanalization was considered complete when all the vessels lumen were patent and partial when there was a decrease of more than 50% in the size of the thrombus and the absence of thrombus extension.
Clinical and imaging data were retrospectively evaluated each three months during the first year and biannually afterwards. The follow-up lasted until the death of the patient or the end of the study (September 2015).
Anticoagulation protocolPrior to anticoagulation, all patients were screened to grade esophageal varices and received primary prophylactic treatment with non-selective beta-blockers or underwent endoscopic therapy in case of intolerance or contraindications to pharmacologic treatment. All patients underwent blood count and tests to evaluate coagulation before treatment. All patients with cirrhosis and PVT were candidates to receive treatment with standard therapeutic dose of enoxaparin, which is administered subcutaneously for 12 months. If there was an underlying thrombophilic disorder or in patients listed for liver transplantation, anticoagulation was continued. Only three patients were treated with vitamin K antagonists (they refused subcutaneous administration of the drug). The target international normalization ratio on these three patients was set between 2 and 3, but attempting to reach as close as possible to 2.5.
Statistical analysisQuantitative variables were expressed as mean±SD or median and range. Qualitative variables were expressed as absolute and relative frequencies. Categorical variables were compared by means of the X2-test and quantitative data were compared with the Student t test. p values <0.05 were considered to indicate statistical significance and all of the tests were two-sided. The statistical software package SPSS 16.0 (SPSS, Inc, Chicago, IL, USA) was used for the analysis.
ResultsThirty-two consecutive patients were evaluated and followed-up for a median of 28.4 months (range 3–65 months). The main patient characteristics are summarized in Table 1. All patients had no symptoms, so the PVT was an incidental finding during routine scheduled ultrasound. PVT was diagnosed with Doppler ultrasound in all the patients. We confirmed PVT with CT in 29 patients (90%) and with MRI in 3 patients (9%). The PVT was partial in 25 patients (78%) and complete in 7 patients (22%). In 3 patients (9%) thrombosis extended to the splenic vein and in 13 patients (40)% to the superior mesenteric vein.
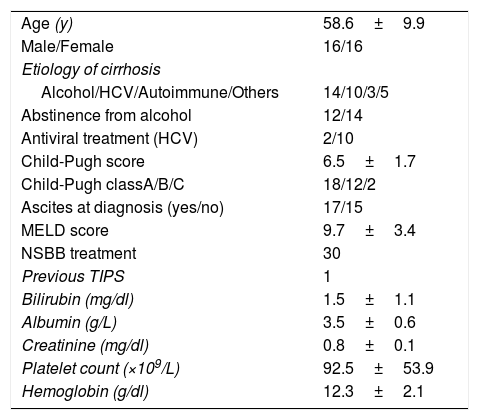
Main baseline characteristics of 32 patients with portal vein thrombosis.
| Age (y) | 58.6±9.9 |
| Male/Female | 16/16 |
| Etiology of cirrhosis | |
| Alcohol/HCV/Autoimmune/Others | 14/10/3/5 |
| Abstinence from alcohol | 12/14 |
| Antiviral treatment (HCV) | 2/10 |
| Child-Pugh score | 6.5±1.7 |
| Child-Pugh classA/B/C | 18/12/2 |
| Ascites at diagnosis (yes/no) | 17/15 |
| MELD score | 9.7±3.4 |
| NSBB treatment | 30 |
| Previous TIPS | 1 |
| Bilirubin (mg/dl) | 1.5±1.1 |
| Albumin (g/L) | 3.5±0.6 |
| Creatinine (mg/dl) | 0.8±0.1 |
| Platelet count (×109/L) | 92.5±53.9 |
| Hemoglobin (g/dl) | 12.3±2.1 |
HCV: hepatitis C virus; MELD: Model for End-Stage liver Disease; NSSB: nonselective beta-blockers.
In 24 patients (75%) a complete thrombophilia screening was done. Four patients (16%) had a thrombophilic disorder: two with factor V Leiden mutation, one with hyperhomocysteinemia and one with JAK2 gene mutation. None of them had another thromboembolic event before or during follow-up. We found no correlation between thrombofilic disorders and recanalization or progression of thrombosis. In all these patients anticoagulation therapy was maintained. One of them (with JAK 2 gene mutation) died after 7 months of treatment and another received liver transplant.
All the patients received anticoagulation treatment. Low-molecular-weight heparin (enoxaparin) was administered to 29 patients and vitamin K antagonists to the remaining three patients. The median interval between time zero and the start of anticoagulation therapy was 2 weeks (range: 0–16 weeks):16 patients (50%) received anticoagulation during the first week, 9 patients (28%) between the second and the third week and 7 patients (22%) after more than three weeks.
Patients were on treatment a median of 13 months (2–45). Overall, 28 patients (87%) received anticoagulation for more than 6 months. Three patients (9%) stopped treatment because of a bleeding complication.
No patient underwent transjugular intrahepatic portosystemic shunt during the study period.
Recanalization was assessed in all patients during follow-up by Doppler ultrasound (each three months).
Recanalization was achieved in 23 patients: complete in 17 patients (53.1%) and partial in 6 patients (18.7%). The median time until achieving this complete response was 7 months (IC 95%: 6–8).
None of the patients had progression of the thrombosis while on anticoagulation.
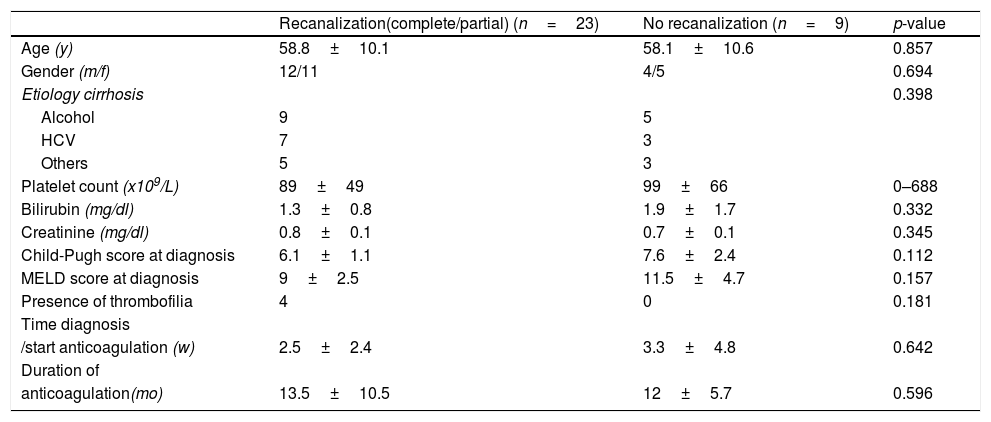
We analyzed possible predictor factors of the recanalization rates (partial or complete), but we did not discover any factor associated with that event. In Table 2 predictor factors of recanalization analyzed are described.
Predictors of portal vein thrombosis response to anticoagulation therapy.
| Recanalization(complete/partial) (n=23) | No recanalization (n=9) | p-value | |
| Age (y) | 58.8±10.1 | 58.1±10.6 | 0.857 |
| Gender (m/f) | 12/11 | 4/5 | 0.694 |
| Etiology cirrhosis | 0.398 | ||
| Alcohol | 9 | 5 | |
| HCV | 7 | 3 | |
| Others | 5 | 3 | |
| Platelet count (x109/L) | 89±49 | 99±66 | 0–688 |
| Bilirubin (mg/dl) | 1.3±0.8 | 1.9±1.7 | 0.332 |
| Creatinine (mg/dl) | 0.8±0.1 | 0.7±0.1 | 0.345 |
| Child-Pugh score at diagnosis | 6.1±1.1 | 7.6±2.4 | 0.112 |
| MELD score at diagnosis | 9±2.5 | 11.5±4.7 | 0.157 |
| Presence of thrombofilia | 4 | 0 | 0.181 |
| Time diagnosis | |||
| /start anticoagulation (w) | 2.5±2.4 | 3.3±4.8 | 0.642 |
| Duration of | |||
| anticoagulation(mo) | 13.5±10.5 | 12±5.7 | 0.596 |
HCV: hepatitis C virus; MELD: Model for End-Stage liver Disease.
Nine patients who achieved a complete recanalization and stopped anticoagulation therapy had rethrombosis (52%). The median time until this event in these patients was 12 months (2–48).
Outcome of clinical eventsOverall, eleven patients (34%) had 19 clinical events during anticoagulation therapy (six patients had more than 1 event). In ten patients these events were liver-related: two variceal bleedings, ten new or worsening ascites and six hepatic encephalopathy. Relative to these events, 35% of patients achieving complete or partial recanalization and 33% of patients not achieving recanalization. We analyzed the relation between recanalization and the presence of hepatic decompensation, but we did not find statistical significance (p=0.5).
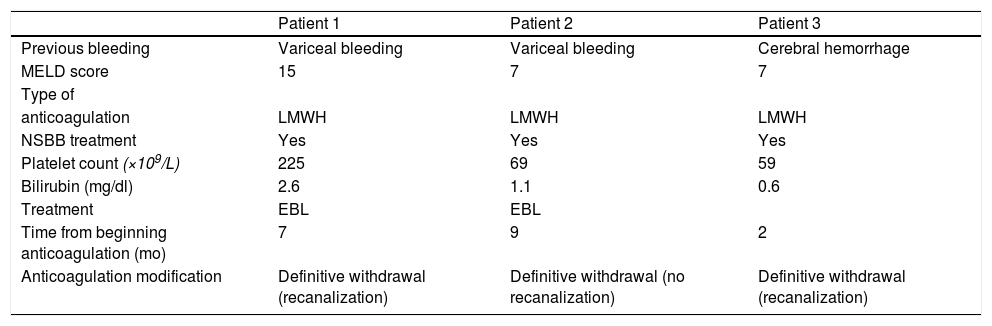
Three patients (9%) had bleeding complications: two variceal bleeding episodes and one cerebral hemorrhage. This last patient had a full recovery without needing rehabilitation. The two patients with variceal bleeding required hospitalization and blood cell transfusion, but they recovered completely. These events happened at 7, 9 and 2 months after starting treatment, respectively (Table 3).
Characteristics of patients with bleeding complications (at time zero).
| Patient 1 | Patient 2 | Patient 3 | |
| Previous bleeding | Variceal bleeding | Variceal bleeding | Cerebral hemorrhage |
| MELD score | 15 | 7 | 7 |
| Type of | |||
| anticoagulation | LMWH | LMWH | LMWH |
| NSBB treatment | Yes | Yes | Yes |
| Platelet count (×109/L) | 225 | 69 | 59 |
| Bilirubin (mg/dl) | 2.6 | 1.1 | 0.6 |
| Treatment | EBL | EBL | |
| Time from beginning anticoagulation (mo) | 7 | 9 | 2 |
| Anticoagulation modification | Definitive withdrawal (recanalization) | Definitive withdrawal (no recanalization) | Definitive withdrawal (recanalization) |
MELD: Model for End-Stage liver Disease; LMWH: low-molecular-weight-heparin; EBL: endoscopic band ligation; NSSB: nonselective beta-blockers.
Nine patients (28%) died: four of liver failure, two of spontaneous bacterial peritonitis and three of liver-unrelated causes (gastric neoplasia, acute pancreatitis and multiorgan failure of unknown origin). Only one of these patients was receiving anticoagulation therapy at the time of death.
In addition to gastric neoplasia, only another patient developed an extrahepatic neoplasia (colo-rectal cancer), one year after PVT.
DiscussionPVT is the most common thrombotic event in cirrhotic patients.18 In spite of the fact that there are no guidelines regarding the management of PVT in cirrhosis and that there are only limited data regarding its safety and benefits, anticoagulation treatment is considered the first-line therapy in these patients. Recently, Baveno VI consensus19 recommends that anticoagulation in cirrhotic patients with PVT should be considered in potential candidates for liver transplant. In our study, all the patients except two were considered candidates for a liver transplantation before starting anticoagulation treatment.
In this regard, there are eight studies published to date about prophylaxis and treatment of non-malignant PVT with anticoagulation therapy in cirrhotic patients.10–16,20 One of these studies evaluated the role of enoxaparin in preventing PVT in patients with advanced cirrhosis. There was no thrombosis in the active arm compared with almost 30% PVT in the control arm.20 The other seven studies published regarding treatment of PVT in cirrhosis included a total of 207 patients.10–16 Treatment (warfarin and/or low-molecular-weight heparin) was associated with complete recanalization rates between 36% and 75%.
In this study, we retrospectively evaluated our experience with anticoagulation therapy in patients with cirrhosis and PVT. Our results show that recanalization is achieved after starting this treatment in a significant percentage of patients. The complete recanalization rate was 53% and partial recanalization was obtained in an additional 18% of patients. These rates of responses are similar to the range reported in previous studies. Recanalization was evaluated by Doppler ultrasound (assessed by two experienced clinicians). The evaluation was made by CT/RNM for uncertain cases. The median time until achieving the complete response was 7 months. None of the patients showed thrombus progression while on anticoagulation. We did not find any factor related to the probability of recanalization. In two studies, the early administration of anticoagulation was associated with it,12,13 but this fact was analyzed but not confirmed in other studies.11,16 In our patients, the median interval between time zero and the start of anticoagulation therapy was 2 weeks – 9 days in the study of Delgado et al.13 Though the time until achieving recanalization is slightly higher to reported in other series, we do not find an explanation, although we do not rule out a possible influence of the early initiation of anticoagulation therapy, as mentioned already, or another factor (not evaluated) as the degree of superior mesenteric vein.16
Thrombosis was partial in most of patients with PVT, according to results from other studies.6
A relevant finding from our series is that more than a half of patients (52%) who had achieved a complete recanalization had a rethrombosis in a relatively short period of time. Amitrano et al.11 and Delgado et al.13 reported recurrent thrombosis rates after stopping anticoagulation of 27% and 38%, respectively. All these results seem to confirm that the rate of response is high in these patients and it is achieved relatively early, so probably anticoagulation therapy should be maintained in the long-term after achieving recanalization, at least in some patients (patients who are candidates for a liver transplantation).
A complete thrombophilia screening was done in two thirds of patients and 20% had a thrombophilic disorder. This prevalence is higher than what has been reported in other series13,14 although the causal role in PVT of these disorders is controversial.6 In any case, we found no correlation between thrombophilic disorders and recanalization or progression of thrombosis.
In a study designed to evaluate the efficacy of enoxaparin in preventing PVT in cirrhotic patients, Villa et al.20 showed this therapy not only may be useful in this situation but decrease the occurrence or recurrence of liver decompensation. Delgado et al.13 described that the number of liver-events during follow-up was lower in the group of patients that achieved recanalization, but the difference did not reach statistical significance. However, other studies did not show any benefit of anticoagulation on decompensation.16 In our study (90% of patients treated with enoxaparin) there were no differences relative to these liver events as well, between patients achieving recanalization, complete or partial, and not achieving recanalization. One reason to explain these results may be, as in other studies,13 the low number of these events.
All previous studies show that anticoagulation appears to be safe with no significantly increased risk of bleeding.21 On the other hand, Cerini et al.22 reported that anticoagulation therapy is not necessarily associated with an increased morbidity/mortality in cirrhotic patients with upper-gastrointestinal bleeding. Our results confirm these results, given that only three patients had bleeding complications. Probably the careful screening and management of varices prior to initiation of anticoagulation has had a great influence in this fact. We did not find any parameter related to higher risk of bleeding (Delgado et al. described that a platelet count below 50×109/L was the only parameter associated with this complication.13).
In conclusion, and despite the limitations of our study (its retrospective nature and the lack of a control group), it shows that anticoagulation therapy in cirrhotic patients with PVT is reasonably safe and it is an effective treatment: 71% patients achieved recanalization (complete in 53%). More than a half of patients who had achieved a complete recanalization had a rethrombosis after stopping anticoagulation. So, these results reinforce the argument for maintaining anticoagulation treatment in the long-term after achieving recanalization, at least in some group of patients (patients who are candidates for a liver transplantation). In any case, it is obvious that this question and other issues about management of PVT should be solved in the near future, in the context of further well-designed prospective trials.
FundingThere was not financial support.
Conflicts of interestThe authors declare no conflicts of interest.