A progressive decrease in Helicobacter pylori eradication rates has been described over the years, driving the need for new antibiotic treatments.
AimTo evaluate the efficacy and safety of the addition of rifaximin (Spiraxin®) to standard triple therapy (omeprazole, amoxicillin and clarithromycin) for the eradication of H. pylori.
MethodsIndependent prospective clinical trial (EUDRACT no.: 2013-001080-23). Forty consecutive adult patients were included with H. pylori infection, dyspeptic symptoms and naive to eradication treatment. A full blood test was performed in the first five patients enrolled to evaluate the safety of the treatment. H. pylori eradication was confirmed with the 13C-urea breath test at least four weeks after the end of treatment with rifaximin 400mg/8h, clarithromycin 500mg/12h, amoxicillin 1g/12h and omeprazole 20mg/12h for 10 days.
ResultsForty patients were consecutively enrolled, 53% woman, mean age 44 years. Indication for eradication: 60% non-investigated dyspepsia, 38% functional dyspepsia and 2% gastric ulcer. Four patients did not attend the eradication confirmatory breath test. The eradication rate was 61% (95% CI: 45–77%) for the protocol and 55% (40–70%) for intention-to-treat. About 76% of the patients experienced adverse events (35% diarrhea, 14% nausea and 24% metallic taste), none of which was serious. The blood tests did not show significant alterations.
ConclusionAcceptable H. pylori eradication rates are not achieved with rifaximin associated with standard triple therapy for 10 days.
Se ha descrito una disminución progresiva de las tasas de erradicación de Helicobacter pylori a lo largo de los años, por lo que se precisan nuevos tratamientos.
ObjetivoEvaluar la eficacia y seguridad de la adición de rifaximina (Spiraxin®) a la triple terapia estándar (omeprazol, amoxicilina y claritromicina) para la erradicación de H. pylori.
MetodologíaEnsayo clínico prospectivo independiente (EUDRA CT: 2013-001080-23). Se incluyeron 40 pacientes consecutivos infectados por H. pylori, con síntomas dispépticos y naïve a tratamiento erradicador. Se realizó un análisis de sangre completo a los 5 primeros pacientes para evaluar la seguridad del tratamiento. La erradicación de la infección se confirmó con la prueba del aliento con 13C-urea al menos 4 semanas tras la finalización del tratamiento con rifaximina 400mg/8h, claritromicina 500mg/12h, amoxicilina 1g/12h y omeprazol 20mg/12h durante 10 días.
ResultadosSe incluyeron 40 pacientes consecutivos, 53% mujeres, edad media 44 años. Indicación para la erradicación: 60% dispepsia no investigada, 38% dispepsia funcional y 2% úlcera gástrica. Cuatro pacientes fueron pérdida de seguimiento y no realizaron el TAU de confirmación. La tasa de erradicación fue del 61% (IC del 95%: 45-77%) por protocolo y del 55% (40-70%) por intención de tratar. El 76% de los pacientes presentó eventos adversos (35% diarrea, 14% náuseas y 24% sabor metálico), ninguno severo o grave. Las analíticas de seguridad no mostraron alteraciones relevantes.
ConclusiónLa rifaximina asociada a la triple terapia estándar durante 10 días no obtiene tasas aceptables de erradicación de H. pylori.
Helicobacter pylori infection constitutes the most important cause of gastric pathologies leading to gastric cancer, therefore, testing and eradicating this infection is recommended.1
H. pylori is susceptible to several antibiotics in vitro, but is much harder to eradicate in vivo.2,3 Since its discovery by Marshall and Warren in 1984,4 different therapies have been tested and used, but these treatments have failed to maintain adequate eradication rates over time.
Nowadays, the most common first line therapy in Europe is a triple regimen based in the combination of a proton pump inhibitor (PPI) and two antibiotics (usually clarithromycin and amoxicillin), representing over 50% of the prescriptions in Europe obtaining suboptimal results (<80%)5; equivalent to results obtained in other regions.6–8
Although the decline in the efficacy of triple therapy, evidenced in other regions, is not clear in Spain, the estimated pooled eradications rate is lower than 80% and therefore should be avoided.9,10 The suboptimal cure rates can be mainly attributed to the increasing bacterial resistances and the poor patient compliance.11 Clarithromycin resistance is considered, by far, the most relevant factor influencing H. pylori eradication.11,12 The most reasonable way to overcome antibiotic resistance would be the use of antibiotics that do not present resistance or with low resistance rates.13
Rifaximin is a synthetic derivate antibiotic from rifamycin, with a similar antibacterial activity but with minimum gastrointestinal absorption.14–16 It has a wide spectrum of action, including Gram-positive and Gram-negative organisms, both aerobic and anaerobic, and intestinal enterobacterias, including H. pylori.17–19 Its bioavailability in the gastrointestinal tract is high, being its minimum inhibitory concentration comparable to that of many other antibiotics used in the eradication of H. pylori.20,21 And because its low absorption, it has a good tolerability profile, and it is not associated with significant drug interaction or with the acquisition of resistance.18,19,22–24
Rifaximin has a known MIC50 of 4μg/ml and MIC90 of 8μg/ml to inhibit H. pylori growth, values found between those of amoxicillin and colloidal bismuth subcitrate,25 which leads to believe it will be a good candidate as H. pylori eradication treatment. In a Spanish study done in 31 strains, all those resistant to clarithromycin were susceptible to rifaximin.20 This entails an added advantage over other antibiotics like metronidazole or clarithromycin.26–28
Up to date, some preliminary studies have been published on the use of rifaximin in the eradication of H. pylori,19–21,29–34 all of them with few patients. Most of the studied therapies that include rifaximin, are dual or triple therapies,19–21,32,33,35 and had obtained eradication rates up to 70%. There is only one clinical trial in which a quadruple therapy with rifaximin (omeprazole, amoxicillin, levofloxacin, and rifaximin) for 7 days34 has been evaluated, obtaining an eradication rate of 80%. This suggests that, to obtain higher rates, rifaximin should prescribed in quadruple therapy for longer for periods (>7 days).
Taking in consideration the high intraluminal concentrations of rifaximin, its activity against H. pylori and its excellent safety profile,22–24 using rifaximin in quadruple therapy by adding it to the triple therapy seems the ideal combination to enhance the “systemic” effect of the antibiotics, overcome the problem of bacterial resistance and improve their eradication efficacy without increasing the adverse effects.
Therefore, the aim of the present study was to evaluate the efficacy and safety of the addition of rifaximin to standard triple therapy for the eradication of H. pylori.
MethodsWe designed a prospective pilot study, open and uncontrolled, in which 40 patients naïve to eradication treatment with dyspeptic symptoms, proven H. pylori infection and who had the triple standard therapy prescribed by routine clinical practice (omeprazole 40mg/12h, clarithromycin 500mg/12h and amoxicillin 1g/12h for 10 days) were enrolled. Exclusion criteria were age below 18, allergic to any of the study drugs, antibiotics or bismuth salts consumption since performing the diagnosis, previous gastric surgery, presence of severe pulmonary disease, hepatic, renal, endocrine, metabolic, or hematologic tumor, chronic advanced disease or any other condition that prevents attending to trial visits and follow up, history of alcohol or drug abuse and patients pregnant or lactating.
To those patients that met the inclusion/exclusion criteria and had signed the informed consent, rifaximin 400mg/8h for 10 days was added to the already prescribed eradication treatment.
A post-treatment follow up visit was scheduled in a period of 15 days after finishing the treatment to verify treatment compliance that was defined as an intake of at least the 90% of the medication. The compliance was verified through a questionnaire and drug accounting by asking the patients to bring all the empty boxes and remaining tablets of all medications that compose the treatment, including the investigational drug. The eradication was assessed with a 13C urea breath test performed at least 4 weeks after the completion of treatment. Adverse effects were assessed in all visits by patient interview as well as variations in the concomitant medication taken during the study. Adverse effects were classified as mild, moderate or severe at the discretion of the physician, following standard definitions. A pregnancy test was performed in all women with child bearing potential.
H. pylori eradication was defined as a negative breath test result, made at least 4 weeks after completion of the eradication therapy. In case that the patient had taken PPIs 2 weeks before the confirmatory test or antibiotics 1 month before the test, breath test was repeated 4 weeks later.
A full blood test (including, blood count, erythrocyte sedimentation rate, hematimetric rates and complete biochemistry) was performed in the first five patients included to evaluate the safety of the treatment.
This study has the approval of the Hospital de La Princesa Ethics Committee and has been conducted in accordance with Good Clinical Practice and the Declaration of Helsinki (Seventh revision, 64th Meeting, Fortaleza, 2013).
Statistical analysisThe proportion and 95% confidence interval (95% CI) was calculated for categorical variables, and the mean±standard deviation for quantitative variables. Analysis of the efficacy of H. pylori eradication was performed on an intention-to-treat basis (including all eligible patients enrolled in the study regardless of compliance with the study protocol; patients with no valuable data were assumed to have been unsuccessfully treated) and on a per-protocol basis (excluding patients whose compliance with therapy was poor and patients with no valuable data after therapy).
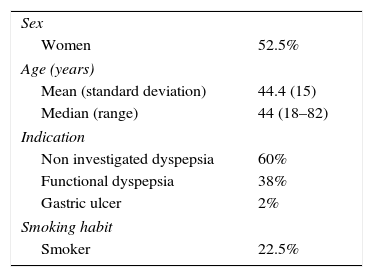
ResultsForty consecutive patients were included (53% women). The average age of the patients included was 44±15 years. The indication for eradication was: 60% non-investigated dyspepsia, 38% functional dyspepsia and 2% gastric ulcer. Twenty-three percent of the patients were smokers. Sixty percent of patients were initially diagnosed using 13C urea breath test, 28% through histology and 13% through the rapid urease test. The characteristics of the study population are described in Table 1.
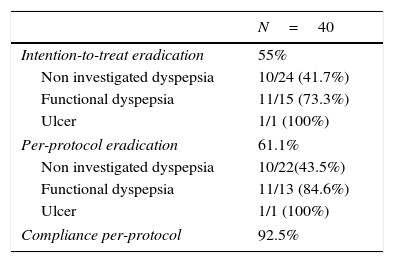
Of the 40 patients enrolled, 4 were lost to follow up and did not perform the confirmation test, therefore compliance could not be verified. Adherence to treatment in patients who attended the post-treatment visit was 100%. Overall adherence was of 93%. The efficacy obtained was 61% (95%CI: 45–77%) per-protocol and 55% (95%CI: 40–70%) by intention-to-treat (Table 2).
Efficacy and compliance.
| N=40 | |
|---|---|
| Intention-to-treat eradication | 55% |
| Non investigated dyspepsia | 10/24 (41.7%) |
| Functional dyspepsia | 11/15 (73.3%) |
| Ulcer | 1/1 (100%) |
| Per-protocol eradication | 61.1% |
| Non investigated dyspepsia | 10/22(43.5%) |
| Functional dyspepsia | 11/13 (84.6%) |
| Ulcer | 1/1 (100%) |
| Compliance per-protocol | 92.5% |
In terms of safety and tolerance, 76% of patients reported adverse effects during treatment and follow-up period, none of them being severe or serious. Seventy-seven percent of the adverse effects were of mild intensity, and 23% were moderate. The most common adverse effect was diarrhea in 35% of patients, followed by metallic taste in 24%, headache in 14%, nausea in 14% and abdominal pain in 2.7% of patients. None of the safety full blood tests presented any significant alteration.
There were no statistical significant differences in eradication rates between sex (p=0.761), smoking habit (p=0.705), diagnosis (p=0.098), compliance (p=0.083) or presence of adverse effects (p=1).
DiscussionUntil now, the definitive treatment for H. pylori eradication remains unclear and although different drug combinations currently exist for the treatment of H. pylori infection, we still do not achieve the ideal 100% eradication rate.35 This is due to poor adherence to treatment (probably due to adverse effects and the duration and complexity of the dosing) and increasing antibiotic resistance, especially to clarithromycin and metronidazole.10,36
So far, the studies evaluating the efficacy of rifaximin, had mainly assessed dual and triple therapies. Dual treatments where rifaximin is combined with omeprazole32 or with clarithromycin, metronidazole or bismuth salts37 have shown eradication rates between 40% and 70%. Triple therapies studied up to date combined rifaximin with a PPI (omeprazole or esomeprazole) and with a second antibiotic (amoxicillin, clarithromycin or levofloxacin) for 7–14 days, obtaining eradication rates between 40% and 85%. Nizhevich et al.35 presented in 2011 a study with 35 children who received a combination of rifaximin and furazolidone or nifuratel for 10 days, with bismuth subcitrate for 14 days, obtaining eradication rates of 85%.
The only published study that contemplates rifaximin in a quadruple therapy34 (omeprazole 20mg, amoxicillin 1g, and rifaximin 400mg twice daily and levofloxacin 200mg twice daily for 7 days) is a double-blind, randomized, controlled clinical trial, that obtained an eradication rate of 80%. The usually (low) recommended duration of treatment and the (low) dose of rifaximin may explain the suboptimal rate obtained.
The results obtained in our study show that quadruple therapy with rifaximin (rifaximin 400mg/8h, clarithromycin 500mg/12h, amoxicillin 1g/12h, and omeprazole 20mg/12h for 10 days) has an efficacy of only 61% by per-protocol, clearly below the efficacy threshold established by consensus conferences (90%). These results do not recommend the addition of rifaximin to triple therapy for the eradication of H. pylori.
The reasons why results were so low rates are not clear. However, one possible explanation is that the highest concentrations of rifaximin in the stomach are reached at 0.5h after ingestion.38 Gastric emptying of 40% or more for liquid meals occurs after 20min39,40 and of 50% for solid meals between 60 and 90min.41 This could prevent reaching of the optimum concentration of rifaximin in the gastric mucus layer where H. pylori is located.42
Finally, although extended periods of treatment, higher doses of rifaximin and a better formulation that enables a prolonged exposure to the drug may increase eradication rates; it is unlikely that it will surpass the results obtained with other traditional quadruple therapies. For this reason, further studies are needed to demonstrate if rifaximin could play a role in H. pylori eradication.
Conflicts of interestDr. Gisbert has served as a speaker, a consultant and advisory member for or has received research funding from MSD, Abbvie, Hospira, Kern Pharma, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma.
Medication was provided free of charge by Bama Geve.
The other authors have no conflict of interest to declare.