In the interferon era, the treatment of hepatitis C virus (HCV) infection in patients on haemodialysis (HD) was limited due to the significant number of treatment-related adverse events (AEs). Direct-acting antivirals (DAAs) have demonstrated their efficacy and safety in the treatment of HCV in patients with advanced chronic kidney disease on haemodialysis. The objective of the study was to evaluate the success in eliminating HCV infection from our dialysis unit using DAAs, and to assess the impact of HCV elimination on clinical and analytical outcomes.
Patients and methodsThis is a prospective, interventional, single-center study at Hospital Clínic de Barcelona. All HCV-RNA positive patients who received antiviral therapy with DAAs within a 3-year period (2014–2017) were analyzed (n=20). Data on virologic response, adverse events, and biochemical and hematological parameters during and after DAA therapy were analyzed.
ResultsAll patients achieved sustained virologic response (SVR) and only 40% of patients presented with mild AEs. None of the patients presented with HCV reinfection after a 1-year follow-up period, and thus HCV was eliminated from our HD unit. SVR was associated with a significant increase in hemoglobin and hematocrit, and a tendency toward the need for lower doses of iron supplementation with no changes in darbepoetin dose.
ConclusionHCV infection can be safely eliminated from HD units with the use of DAAs, preventing new infections in patients and healthcare staff. In the short term, the achievement of SVR is associated with an improvement in the control of anemia.
En la época del interferón, el tratamiento del virus de la hepatitis C (VHC) en pacientes en hemodiálisis (HD) se veía limitado por la presencia de efectos adversos relacionados con el tratamiento. Los agentes antivirales directos (AAD) han demostrado ser seguros y eficaces en el tratamiento del VHC en pacientes con insuficiencia renal crónica en hemodiálisis. El objetivo del estudio fue evaluar el éxito en eliminar la infección por VHC de nuestra unidad de diálisis con el uso de AAD, y determinar el impacto clínico y analítico de la curación de la infección.
Pacientes y métodosPara ello se realizó un estudio prospectivo de intervención en el Hospital Clinic de Barcelona y su centro de diálisis. Se incluyeron todos los pacientes (n=20) con ARN-VHC positivo que recibieron tratamiento antiviral con AAD durante un periodo de 3 años (2014-2017). Se analizaron los datos de respuesta virológica, efectos adversos y parámetros hematológicos y bioquímicos durante y después del tratamiento.
ResultadosTodos los pacientes alcanzaron una respuesta viral sostenida (RVS) y solo una 40% presentaron efectos adversos leves. Ningún paciente presentó reinfección por el VHC y por ello tras un año de seguimiento se consideró la eliminación del VHC de nuestra unidad de diálisis. La RVS se asoció con aumento significativo de la hemoglobina y el hematocrito, y una tendencia a la necesidad de una dosis más baja de suplemento de hierro sin cambios en la dosis de darbepoetina.
ConclusiónCon la utilización de AAD, la infección por el VHC puede ser eliminada de forma segura de las unidades de diálisis, previniendo la transmisión de la infección a pacientes y personal sanitario. A corto plazo, la RVS se asoció con una mejoría en el control de la anemia.
Recent data estimates that the global prevalence of hepatitis C virus (HCV) infection is around 1.6% with a viremic prevalence of 1.1%. This corresponds to 80 million people infected with HCV worldwide.1 Hepatitis C infection is a common cause of liver disease in patients with chronic kidney disease (CKD) who receive long-term renal replacement therapy and it is associated with an increased risk of death, hospitalization, anemia and a reduced quality of life.2–4 Dialysis Outcomes and Practice Pattern Study (DOPPS) showed a prevalence of HCV infection in hemodialysis (HD) patients of 7.5%.4 However, some studies suggest that the prevalence may be underestimated due to the presence of occult HCV infection, defined as the presence of HCV-RNA in hepatocytes and absence of anti-HCV antibodies in routine serological tests.3 The risk of infection varies according to the type of renal replacement therapy (being higher in patients treated with maintenance HD), the time on HD, and the prevalence of HCV infection in the specific dialysis unit.3,5
Chronic HCV infection in HD patients is associated with an increase in mortality rate not only due to liver causes, but also related with cardiovascular disease, anemia, hepatitis B virus (HBV) or human immunodeficiency virus (HIV) coinfection, and essential mixed cryoglobulinemia.3,5–7 In addition, HCV infection contributes to an increase morbidity and mortality after kidney transplantation. Indeed, hepatitis C positive kidney transplantation recipients are at increased risk of developing de novo and recurrent membranous nephropathy, membranoproliferative glomerulonephritis and transplant glomerulopathy.8
Anemia is a common complication of patients with chronic kidney disease. Symptoms generally occur when hemoglobin levels are below 10g/dL, but can be more severe when kidney function worsens.9 More importantly, anemia has been associated with an increased mortality and a reduced quality of life.10 There is a scarcity of information on the role of HCV and CKD-related anemia, but there are some data showing that HCV positive patients have low hemoglobin levels and higher EPO dose requirements compared to uninfected patients.11 However, the impact of HCV infection elimination on the control of anemia is unknown.
New direct-acting antivirals (DAAs) have demonstrated their efficacy and safety on the treatment of HCV in patients with advanced CKD on HD, with sustained virological response (SVR) rates ranging between 90% and 100% and excellent safety data.12–15 Therefore, we hypothesized that HCV infection can be safely eliminated from HD units in order to avoid the risk of new HCV infections among HD patients and staff and that HCV elimination could impact on renal function and CKD-related complications. The aims of the study were to determine the success in eliminating HCV infection from our dialysis unit after DAAs therapy in all patients on HD, and to evaluate the impact of therapy on clinical and analytical outcomes.
Material and methodsStudy design and antiviral therapyThis is a prospective, observational, single center study, in which all HCV-RNA positive patients in our HD unit (at Hospital Clínic Barcelona and its associated dialysis centers) received antiviral therapy with an interferon (IFN)-free regimen within a 3 year period (April 2014 to March 2017). The antiviral regimen was decided by the treating physician based on published guidelines and information available at the time of treatment indication (Asunaprevir [ASV]+Daclatasvir [DCV], Ombitasvir/Paritaprevir/ritonavir with or without Dasabuvir [3D or 2D], Grazoprevir [GZR]/Elbasvir [EBV], or Sofosbuvir [SOF]+DCV). Direct-acting antivirals were administered according to the recommendations of the specific insert packages. The usage and dose of RBV were also decided by the treating physician according to the treatment regimen recommendations and the status of the patient. The duration of therapy was 12 or 24 weeks according to recommendations of the European Association for the Study of the Liver (EASL) guidelines.16 Since the aim of the study was the elimination of HCV infection from HD unit, the presence of significant comorbidities was not considered as a contraindication to receive antiviral therapy. Potential drug–drug interactions were actively investigated and if detected, concomitant medications were modified appropriately.
MonitoringFor each patient, data on age, gender, previous comorbidities, time on dialysis, duration of each dialysis session, etiology of CKD, HBV or HIV coinfection, and previous HCV treatments were collected at baseline. Liver tests (aspartate aminotransferase [AST], alanine aminotransferase [ALT], gamma glutamyl-transpeptidase [GGT], alkaline phosphatase [ALP] and bilirubin), inflammatory markers (C-reactive protein, CRP) and parameters for the control of anemia (hematocrit, hemoglobin, ferritin, transferrin saturation index [TSI], dose of darbepoetin and intravenous iron) were monitored monthly from 3 months before starting antiviral therapy to the end of the follow-up (12 months after treatment discontinuation). Liver stiffness measurements (LSM) were performed at baseline and 3 months after treatment discontinuation. Both determinations were conducted after HD session to avoid the impact of fluid overload on LSM.17
HCV-RNA was measured by quantitative polymerase chain reaction with a lower limit of quantification of 15YIU/L (Versant kPCR Molecular Systems, Siemens Medical Solutions Diagnostics, New York, USA) at baseline and week 4 of antiviral therapy, end of therapy and 12 weeks after treatment discontinuation. Afterwards, HCV-RNA was monitored every 3 months according to local protocol at the HD unit. Sustained virological response (SVR) was considered when HCV-RNA was negative 12 weeks after the end of treatment.
Side effects were assessed during treatment and within 12 months of treatment discontinuation. All adverse events referred by the patient were collected. Special attention was given to the development or worsening of anemia (defined as hemoglobin levels<10g/dL). The management of anemia was carried out by correcting the dose of darbepoietin and intravenous iron according to hemoglobin, hematocrit, ferritin, and transferrin saturation index (TSI).18
Statistical analysisContinuous variables are expressed as mean (standard deviation). Categorical variables are expressed as number and percentage. Comparisons between categorical variables were performed by Chi-square test or Fischer test as appropriate. Comparisons between continuous variables were performed by T-test or non-parametric tests. A one-way repeated measures ANOVA was used to determine whether there was a statistical difference between paired analyses. A p value <0.05 was considered significant. Statistical analysis was performed using the statistical SPSS version 23.
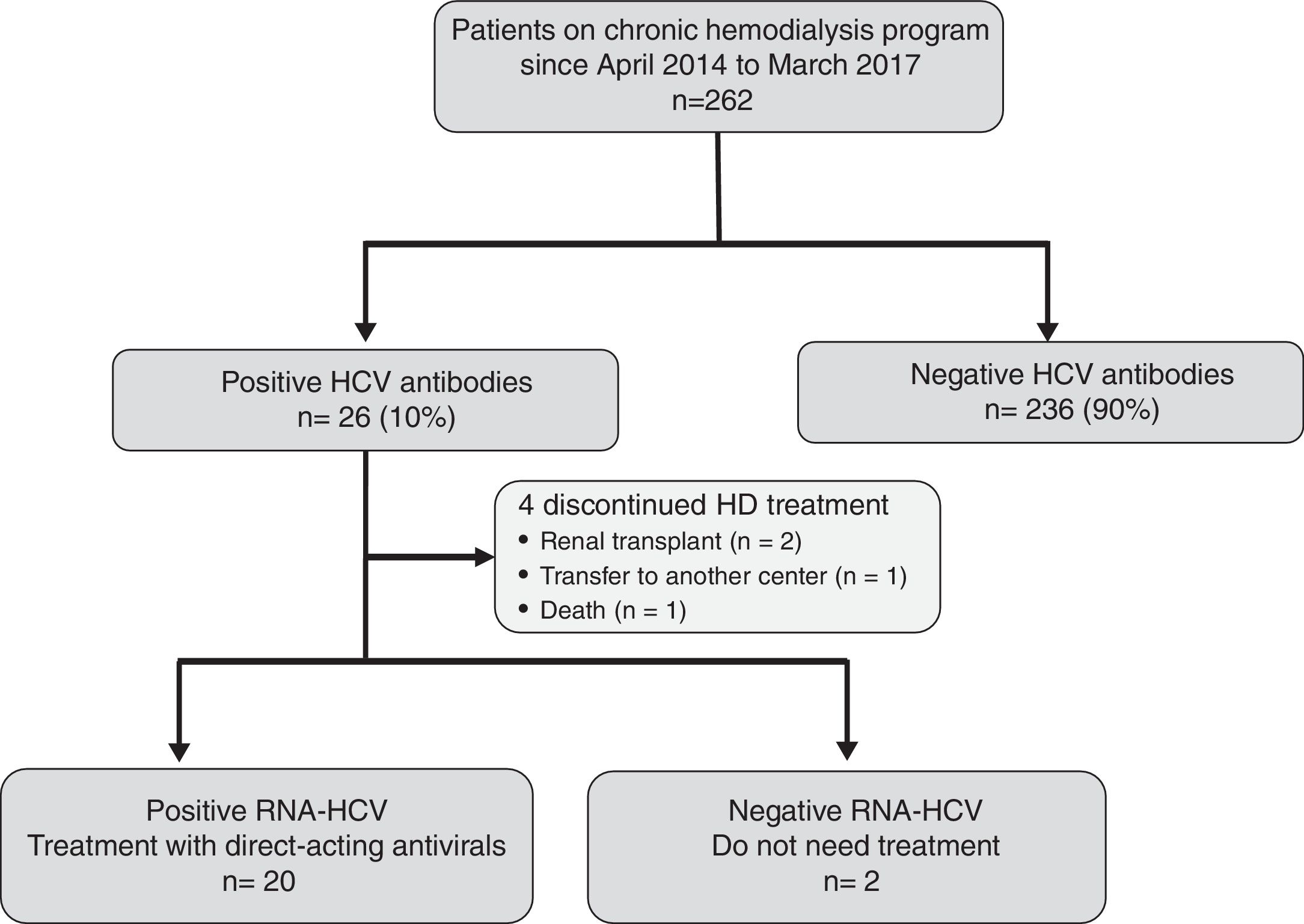
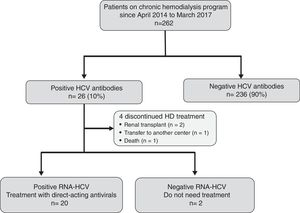
ResultsEffectiveness and tolerability of antiviral therapyAs shown in Fig. 1, 26 (10%) out of the 262 attending our HD unit during the study period were anti-HCV and HCV-RNA positive. During the study period, 4 patients discontinued HD treatment (2 due to kidney transplantation, 1 patient was transfer to another center, and 1 patient died due to hepatocellular carcinoma), and 2 patients did not require antiviral treatment due to spontaneous HCV-RNA negativization. Thus, 20 patients were candidates to receive DAAs treatment.
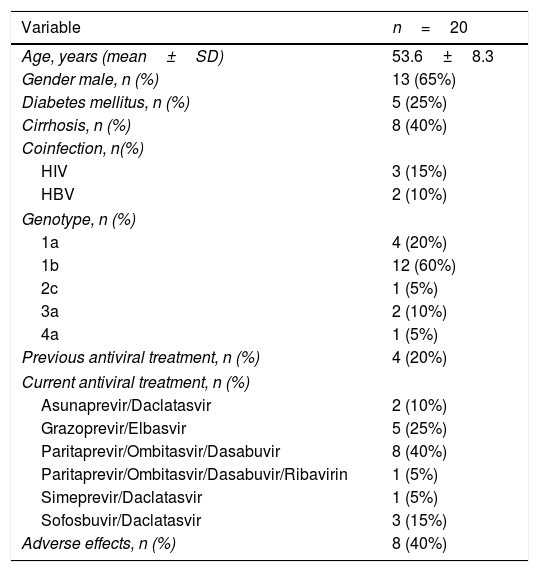
The characteristics of the 20 treated patients are shown in Table 1. Briefly, 65% were male, with a mean age of 53.6±8.3 years, infected with genotype 1b (60%), and stable on HD over a period of 82±79 months. Twenty percent of patients were non-responders to a previous course of an IFN-based therapy. The dialysis modality was on-line hemodiafiltration with post-dilutional infusion in all patients. Twenty-five percent (n=5) of patients had diabetes mellitus, 40% (n=8) had cirrhosis and 25% (n=5) had other viral coinfections (3 HIV infection, 2 HBV infection). The etiologies of CKD were: glomerulonephritis (n=8, 40%), diabetic nephropathy (n=3, 15%), amyloidosis (n=2, 10%), chronic interstitial nephropathy (n=2, 10%), nephroangiosclerosis (n=1, 5%), obstructive uropathy (n=1, 5%). In the remaining 3 patients (15%) the etiology of CKD was unknown.
Baseline characteristics of the patients included in the study.
| Variable | n=20 |
|---|---|
| Age, years (mean±SD) | 53.6±8.3 |
| Gender male, n (%) | 13 (65%) |
| Diabetes mellitus, n (%) | 5 (25%) |
| Cirrhosis, n (%) | 8 (40%) |
| Coinfection, n(%) | |
| HIV | 3 (15%) |
| HBV | 2 (10%) |
| Genotype, n (%) | |
| 1a | 4 (20%) |
| 1b | 12 (60%) |
| 2c | 1 (5%) |
| 3a | 2 (10%) |
| 4a | 1 (5%) |
| Previous antiviral treatment, n (%) | 4 (20%) |
| Current antiviral treatment, n (%) | |
| Asunaprevir/Daclatasvir | 2 (10%) |
| Grazoprevir/Elbasvir | 5 (25%) |
| Paritaprevir/Ombitasvir/Dasabuvir | 8 (40%) |
| Paritaprevir/Ombitasvir/Dasabuvir/Ribavirin | 1 (5%) |
| Simeprevir/Daclatasvir | 1 (5%) |
| Sofosbuvir/Daclatasvir | 3 (15%) |
| Adverse effects, n (%) | 8 (40%) |
SD: standard deviation.
The most commonly used DAAs regimen was Ombitasvir, Paritaprevir/ritonavir and Dasabuvir (n=9; 45%). Ribavirin (RBV) was added only in one patient infected with genotype 1a. Interestingly, 3 patients infected with genotypes 2 or 3 received a Sofosbuvir-based combination (off-label). The specific treatment, dose and duration are shown in Supplementary Table 1.
At week 4 of therapy, viral load for HCV was undetectable in all patients and all of them achieved SVR. No reinfections were observed in any of the subjects during the follow-up period. After achieving SVR, 3 patients who asked for it were relocated in dialysis shifts for HCV negative patients.
Medication was well tolerated with mild side effects in 40% of patients (Table 3). None of these side effects led to treatment discontinuation.
Impact of therapy on clinical and analytical outcomesAs expected, SVR was associated with an improvement in liver tests (Supplementary Table 2) and liver stiffness measurement determined at the time of SVR (from 9.7±5.1kPa to 7.5±4.0kPa; p=0.008). C-reactive protein did not change significantly changed during and after DAA therapy (Supplementary Table 2).
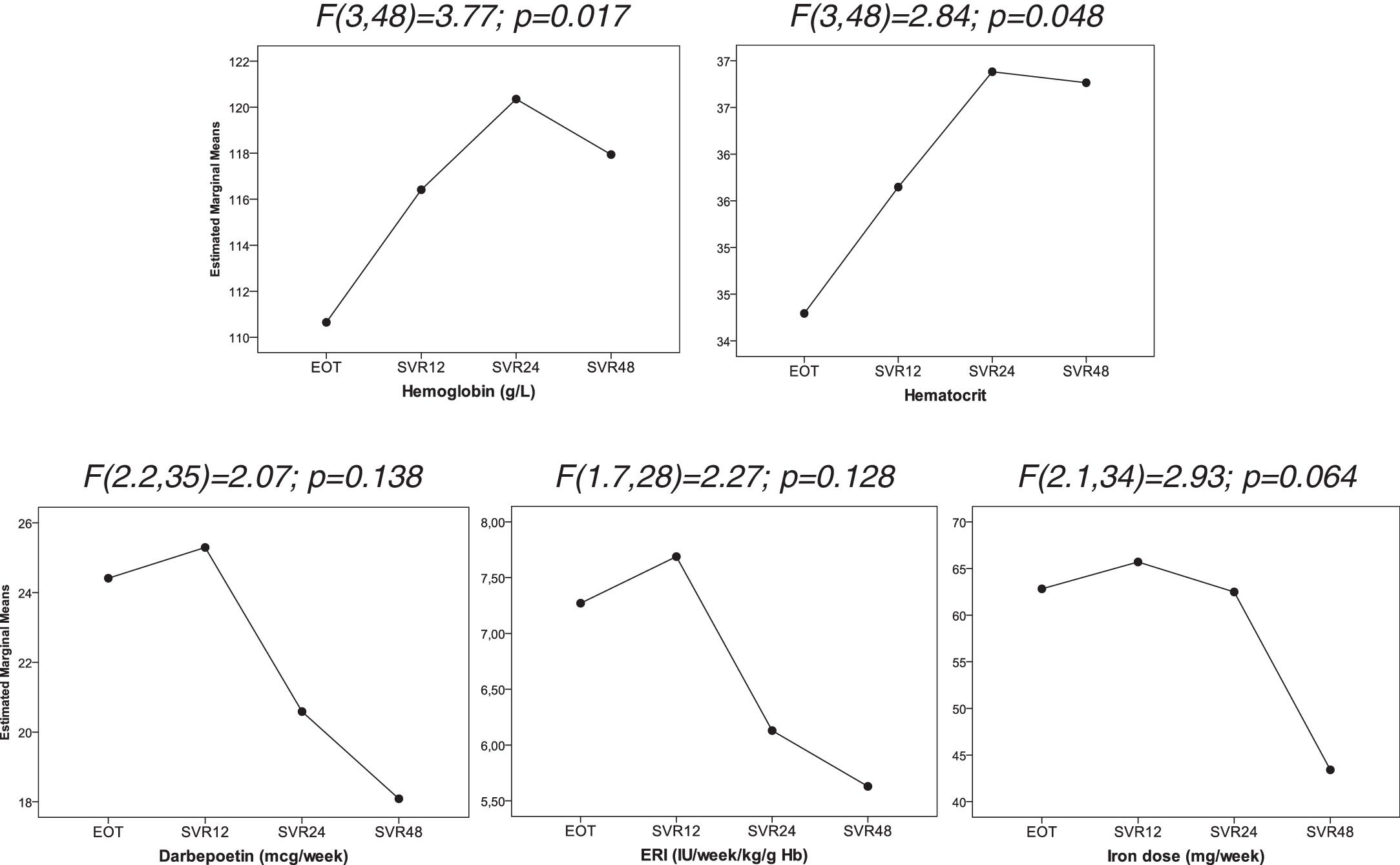
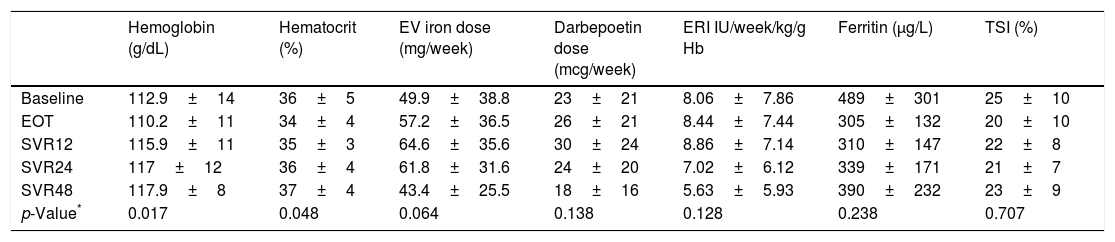
We observed an improved control of anemia after DAAs treatment (Fig. 2), with a significant increase hemoglobin and hematocrit levels after the end of treatment (p=0.017 and p=0.048; respectively). This was associated with a tendency toward a decrease in the dose of intravenous iron (p=0.064). There were no significant changes in the dose of darbepoetin and ERI index. In addition, ferritin levels and transferrin saturation index did not significantly changed after treatment discontinuation (Table 2).
Evolution of hematological parameters after treatment discontinuation. Data were analyzed using one-way repeated measures ANOVA. EOT: End of therapy, SVR12: 12 weeks after treatment discontinuation, SVR24: 24 weeks after treatment discontinuation, SVR48: 48 weeks after treatment discontinuation.
Hematological parameters during and after treatment discontinuation.
| Hemoglobin (g/dL) | Hematocrit (%) | EV iron dose (mg/week) | Darbepoetin dose (mcg/week) | ERI IU/week/kg/g Hb | Ferritin (μg/L) | TSI (%) | |
|---|---|---|---|---|---|---|---|
| Baseline | 112.9±14 | 36±5 | 49.9±38.8 | 23±21 | 8.06±7.86 | 489±301 | 25±10 |
| EOT | 110.2±11 | 34±4 | 57.2±36.5 | 26±21 | 8.44±7.44 | 305±132 | 20±10 |
| SVR12 | 115.9±11 | 35±3 | 64.6±35.6 | 30±24 | 8.86±7.14 | 310±147 | 22±8 |
| SVR24 | 117±12 | 36±4 | 61.8±31.6 | 24±20 | 7.02±6.12 | 339±171 | 21±7 |
| SVR48 | 117.9±8 | 37±4 | 43.4±25.5 | 18±16 | 5.63±5.93 | 390±232 | 23±9 |
| p-Value* | 0.017 | 0.048 | 0.064 | 0.138 | 0.128 | 0.238 | 0.707 |
Data are expressed as median (range). EOT: end of therapy, SVR12: 12 weeks after treatment discontinuation, SVR24: 24 weeks after treatment discontinuation, SVR48: 48 weeks after treatment discontinuation.
Adverse events reported during antiviral therapy.
| Patient number | Adverse event |
|---|---|
| Patient 1 | No |
| Patient 2 | Arthralgia |
| Patient 3 | Heartburn |
| Patient 4 | No |
| Patient 5 | No |
| Patient 6 | Diarrhea |
| Patient 7 | Diarrhea and pruritus |
| Patient 8 | No |
| Patient 9 | Pruritus |
| Patient 10 | No |
| Patient 11 | No |
| Patient 12 | No |
| Patient 13 | No |
| Patient 14 | No |
| Patient 15 | No |
| Patient 16 | Pruritus |
| Patient 17 | Headache |
| Patient 18 | No |
| Patient 19 | No |
| Patient 20 | No |
Here, we showed for the first time that with the new DAAs treatments it is possible to eliminate HCV infection from HD units. Despite the prevalence of HCV in patients on HD having declined significantly in recent years, it is still higher than that of the general population carrying a significant increase in morbidity and mortality.19,20 The international observational study on the long-term guidelines and results of patients on HD (DOPPS) reviewed 76,689 adult patients between 1996 and 2015.4 The prevalence of HCV infection observed among countries participating in all phases of DOPPS was 14.3% in phase 1 (1996–2001), 10.7% in phase 2 (2002–2004), 8.8% in the phase 3 (2005–2008), 6.8% in phase 4 (2009–2011) and 8.7% in phase 5 (2012–2015). The prevalence of HCV infection in our HD unit, with a mean time in dialysis of 82.0±78.7 months was 10%, which is slightly higher than that observed in DOPPS phase 5.
Prior to 2011, standard HCV treatment was based on pegylated interferon (PegINF) or the combination of PegIFN and RBV, which resulted in SVR rates of 40–50%, although their significant adverse effects and low rate of SVR, limited their widespread use. Despite the relatively good results in terms of efficacy in patients on HD treated with PegIFN and RBV,21 the use of this therapy was hampered by the significantly high number of adverse events observed in this subpopulation. A study by our group22 showed that up to 60% of patients on HD treated with an IFN-based therapy early discontinued treatment due to adverse events (including hematological disorders and infections). This explains the low applicability of IFN-based therapies in patients on HD as shown in the DOPPS study, in which only 1.5% of the patients included received antiviral therapy.4
The development of IFN-free therapies with different combinations of DAAs has revolutionized the treatment of patients in HD. There are 3 clinical trials specifically designed to evaluate the safety and efficacy of antiviral therapy in patients with chronic kidney disease stages 4 and 5. The RUBY-112 trial evaluated the 3D combination with or without RBV in 20 genotype 1-infected patients with CKD stages 4 and 5. Overall, 90% (18 out of the 20 patients) achieved SVR12. Anemia was the most common adverse event. In the second part of the study, 18 genotype 1a and 4 infected patients (normally requiring RBV) received the 3D or 2D combination without RBV and all of them achieved SVR. In the C-SURFER13 study, 224 genotype 1 infected patients were randomly assigned to receive the combination of Grazoprevir and Elbasvir once daily for 12 weeks or placebo (deferred treatment). In the full set analysis, 94% and 95% of patients in the immediate and the deferred treatment groups, respectively, achieved SVR12. More recently, the combination of Glecaprevir and Pibrentasvir administered for 12 weeks to 104 patients infected with different HCV genotypes and CKD stages 4 and 5 achieved an SVR of 100% with excellent safety results.14
Similar to the results of clinical trials or real life cohorts,12,23,24 we obtained excellent results with the different DAAs combinations administered to our cohort. Most of our patients received the 3D combination for 12 weeks and all of them achieved SVR. Only one patient (genotype 1a) required RBV and prolonged treatment duration (24 weeks) because of the presence cirrhosis. Despite Sofosbuvir is currently not approved for the treatment of patients with a glomerular filtration rate<30ml/min, 3 of our patients (infected with genotype 2 and 3) with no other treatment options received Sofosbuvir in combination with Daclatasvir for 12 or 24 weeks with excellent results in terms of efficacy and safety. This is similar to what has been observed in small series of cases published recently evaluating different combinations of Sofosbuvir-based regimes in which SVR rate ranged between 67% and 100% depending on the dose of Sofosbuvir (standard dose of 400mg per day vs. 400mg every other day or 200mg per day) and the additional drug (RBV vs. other DAA).20,25–27 More importantly, despite the concerns of using Sofosbuvir in patients on HD due to the potential side effects secondary to GS331007 (principal metabolite of Sofosbuvir) accumulation, we and others did not observe serious adverse events in patients on HD receiving this drug. However, more data are needed to confirm these results and to recommend the widespread use of Sofosbuvir in patients on HD.
Interestingly, we observed an improvement in the control of anemia with a significant increase in hemoglobin and hematocrit levels, and a tendency toward a decrease in the dose of intravenous iron during the 12 months after treatment discontinuation. The explanation for this phenomenon is unknown. However, one can speculate that elimination of HCV infection could improve iron metabolism and response to erythropoiesis due to a decrease in systemic inflammation. The role of HCV infection on iron metabolism in patients on HD is controversial. A study by Abdalla et al.,11 showed that HCV positive patients on HD had higher EPO requirement (49.6 vs. 14.4 units/kg/dialysis; p=0.006) and low hemoglobin levels (10.5 vs. 11.2g/dL; p=0.03) as compared to HCV negative patients. However, more recent studies have shown opposite results. For example, Khurana et al.,28 found that HCV positive patients required low EPO dose as compared to HCV negative counterparts (253 vs. 460 units/kg/month) with no significant differences in other parameters. In addition, the number of patients not requiring EPO was significantly higher among HCV positive patients. Since, CKD-related anemia is associated with significant morbidity and mortality; the improvement in the control of anemia could improve cardiovascular risk, long-term survival, and quality of life. Obviously, the limited number of patients included in the study and the short follow-up duration (up to 1 year after treatment discontinuation) preclude us to draw a formal conclusion. However, new studies, with longer follow-up of treated patients, would be interesting to confirm this hypothesis.
In order to prevent HCV transmission in HD units, KDIGO (Kidney Disease Improving Global Outcomes) guidelines recommend to strictly adhering to infection-control procedures. However, isolation of HCV infected patients or using specifically dedicated machines for these patients is not recommended.29 In our unit, we follow these recommendations but all HCV positive patients are located in dialysis shifts with a specific nurse devoted to their care following the international recommendations to avoid blood-borne pathogens. There is no information on the literature regarding the management of HD patients achieving SVR after antiviral therapy. In our unit viral elimination, at the time of SVR achievement (3 months after the end of therapy) patients are relocated to HCV negative dialysis shift.
Our study has several limitations, including the low number of patients enrolled, the absence of patients infected with less frequent genotypes, and the lack of a standardized treatment for all patients due to the rapid evolution of antiviral treatments within the past few years. However, this is a proof of concept that HCV infection can be eliminated from HD units by treating all patients (including patients with severe comorbidities) by a multidisciplinary team of experts in the management of this subpopulation.
In conclusion, with the incorporation of new DAAs it is possible to eradicate HCV from dialysis units. The variety of therapeutic possibilities, along with the high efficacy and good tolerance of these drugs allows treating and curing the whole dialysis population. HCV elimination in HD units is a major breakthrough that should allow a zero incidence of hepatitis C infections. Despite further research and validation is required, it appears that HCV elimination is associated, at individual level, to an improvement in the control of anemia.
Conflict of interestMCL has received advisory fees from Janssen, Abbvie, Gilead, MSD and BMS. The other authors have declared no conflict of interest.