Direct-acting antivirals for the treatment of hepatitis C virus (HCV) represented a paradigm shift. In 2017, sofosbuvir/velpatasvir (SOF/VEL-Epclusa®) was approved, which showed a high cure rate in all patient, contributing to HCV elimination. The analysis aimed to quantify the clinical and economic value of SOF/VEL in HCV chronic patients since its approval in Spain.
MethodsAn economic evaluation was elaborated adapting a Markov model that simulated the lifetime disease progression in of all HCV chronic patients treated with SOF/VEL (30,488 patients) since its launch (5-years), compared to previous therapies. Patients entered the model and were distributed between the fibrosis states (F0-to-F4) in treated and untreated. All patients (100%) were treated with SOF/VEL regardless of their fibrosis, and 49% with previous therapies in ≥F2. The average sustained viral response (SVR) rates 98.9% SOF/VEL versus 61.0% previous therapies. All parameters for the analysis were obtained from real-life data and literature. Only direct healthcare costs associated with disease management were included. The SOF/VEL value was measured as the number of hepatic complications avoided and their associated cost, and hepatic mortality compared to previous therapies. National Health System perspective and a 3% discount rate was applied.
ResultsSOF/VEL decreased the number of liver complications, avoiding 92% decompensated cirrhosis, 80% hepatocellular carcinomas, and 87% liver transplants, as well as 85% liver-related mortality. Their cost associated was reduced, amounting to savings of 197M€.
ConclusionSOF/VEL adds relevant value to the HCV treatment, reducing the clinical and economic disease burden and contributing to HCV elimination in Spain.
Los antivirales de acción directa para el tratamiento del virus de la hepatitis C (VHC) representaron un cambio de paradigma. En 2017 se aprobó sofosbuvir/velpatasvir (SOF/VEL-Epclusa®), con elevadas tasas de curación en todos los pacientes, contribuyendo a la eliminación VHC. El objetivo fue evaluar el valor clínico y económico de SOF/VEL en pacientes crónicos desde su aprobación en España.
MétodosSe adaptó un modelo de Markov que simulaba la progresión de la enfermedad durante toda la vida de pacientes crónicos tratados con SOF/VEL (30.488 pacientes) desde su lanzamiento (5 años), versus terapias previas. Los pacientes entraron al modelo distribuidos entre los estados de fibrosis (F0-F4) en tratados y no tratados. Todos los pacientes fueron tratados con SOF/VEL independientemente de su fibrosis, y el 49% con terapias previas (≥F2). La respuesta viral sostenida promedio: 98,9% SOF/VEL versus 61,0% terapias previas. Los parámetros para el análisis se obtuvieron de datos reales y literatura. Solo se incluyeron costes sanitarios directos asociados al manejo de la enfermedad. El valor SOF/VEL se midió como el número de complicaciones hepáticas evitadas y su coste, y la mortalidad hepática versus terapias previas. Se aplicó la perspectiva del Sistema Nacional de Salud y una tasa de descuento (3%).
ResultadosSOF/VEL disminuyó las complicaciones hepáticas, evitando el 92% cirrosis descompensadas, el 80% hepatocarcinomas y el 87% trasplantes hepáticos, así como el 85% la mortalidad hepática; generando ahorros de 197 millones de euros.
ConclusionesSOF/VEL añade un valor relevante al tratamiento del VHC, reduciendo la carga clínica y económica de la enfermedad y contribuyendo a la eliminación VHC en España.
Chronic hepatitis C is a liver disease caused by the hepatitis C virus (HCV) that affects 56.8million people worldwide.1 Although the prevalence of the viremia has decreased in recent years, it is estimated that in Europe it is 1.2%1 and in Spain, between 0.22 and 0.3%.2 The infection can remain in mild states of fibrosis for years, but a high percentage of patients develop cirrhosis, which is associated with the progression of the disease to more severe states, such as decompensation and hepatocellular carcinoma.1 Hepatitis C is one of the main causes of liver death.3 Hepatocellular carcinoma is the most common type of cancer in adults caused by prolonged chronic liver disease and has been linked to different risk factors, such as infection by hepatitis virus and cirrhosis.4
Chronic hepatitis C represents a substantial economic burden, mainly associated with liver complications, which are added to other complications related to comorbidity5 and extrahepatic manifestations.6 Hepatitis C patients often have some extrahepatic manifestations in the course of chronic infection, such as non-Hodgkin lymphoma and cardiovascular and neurological diseases, among others.7
Treatment for hepatitis C has evolved in recent years such that current treatments achieve a significant decrease in liver morbidity and mortality.8 Initially, patients were treated with pegylated interferon and ribavirin, a treatment administered intravenously but that was associated with significant side effects and with low rates of sustained virological response (SVR).9 Later, pegylated interferon was combined in double or triple therapy with telaprevir and/or boceprevir, and although the response rates improved, it could not be administered to all patients due to its clinical characteristics.10 In 2015, with the commercialization of sofosbuvir, oral direct-acting antivirals (DAAs) began to appear on the market. DAAs block the replication of the virus, with a short duration of treatment, high response rates, and minimal adverse events.11 Most recently, sofosbuvir/velpatasvir (SOF/VEL) has further improved the therapeutic arsenal and is one of the standard treatments for HCV disease.11,12 According to the clinical guidelines, SOF/VEL is a treatment that is effective against all genotypes of HCV (pangenotypic) and can be administered successfully to a wide spectrum of patients, even those who are considered difficult to treat. Patients with decompensated cirrhosis are among them. In addition, DAAs have simplified the diagnosis and follow-up of chronic patients, which has an impact on the optimization of health resources for the management of the disease.13
Current treatments have helped change our outlook on HCV disease in the pursuit of its eradication.14 The objective of this analysis was to quantify the clinical and economic impact of treatment with Epclusa® (sofosbuvir/velpatasvir) of patients with chronic hepatitis C in Spain during the 5 years since its launch.
MethodsAn economic evaluation is carried out following the method of previous studies15 to estimate the impact on mortality and the number of clinical events associated with liver disease, as well as their costs, comparing a simulated cohort of patients treated with SOF/VEL versus the same cohort treated with therapies prior to DAA drugs (peginterferon and ribavirin in double/triple therapy with telaprevir or boceprevir). The patient cohort is estimated from the total number of patients treated from 2017 to 2022 with SOF/VEL, totalling 30,488.16,17 The estimated mean age is 45 years, and the degree of fibrosis of the patients varies during the first 5 years adjusting to real-life data (ranges: 3.9–52.9% for F0–F1, 12.0–27.5% for F2, 3.0–17.8% for F3, 20.9–23.6% for F4).16 In the SOF/VEL cohort, 100% of the patients are treated regardless of the fibrosis stage, with SVR rates of 99.4% (F0–F2), 99.6% (F3), and 97.9% (F4).18 In the cohort treated with previous therapies, in clinical practice, only patients with fibrosis≥F2 (49%) are treated, who have SVR rates between 60.6% and 61.2%.13
The progression of chronic hepatitis C throughout the life of the patients (time horizon) was simulated using a previously published Markov model.13 The model is composed of different health states—fibrosis (F0, F1, F2, F3, F4)—and their corresponding SVR, decompensated cirrhosis, hepatocellular carcinoma, liver transplantation, post-transplantation, and mortality rates (liver-related and all-cause). The probabilities of transition between states are the same as in the published model.13 In the analysis, patients enter the model during the first 5 years (22% year 1, 26% year 2, 22% year 3, 13% year 4, 17% year 5)16,17 proportionally distributed in the different states of fibrosis and distributed between treated and untreated. Treated patients can achieve SVR or progress to other health states, depending on the course of the disease. Untreated patients progress according to the natural history of chronic hepatitis C. Patients can transition to the state of death due to a hepatic cause from the HCV or another cause (all-cause death).
The analysis was carried out from the perspective of the National Health System (NHS), considering only those direct healthcare costs derived from clinical events in the liver, not including the cost of treatment. The same annual costs are used as in the previous model,13 but they are updated with the interannual variation of the published Consumer Price Index (€, 2022).19
For each treatment, two types of results were obtained: (a) clinical, measured as the numbers of liver deaths and liver clinical events (decompensated cirrhosis, hepatocellular carcinoma, liver transplantation); and (b) economic, estimating the costs associated with the clinical events produced. For both results, a discount rate of 3% was assumed.20
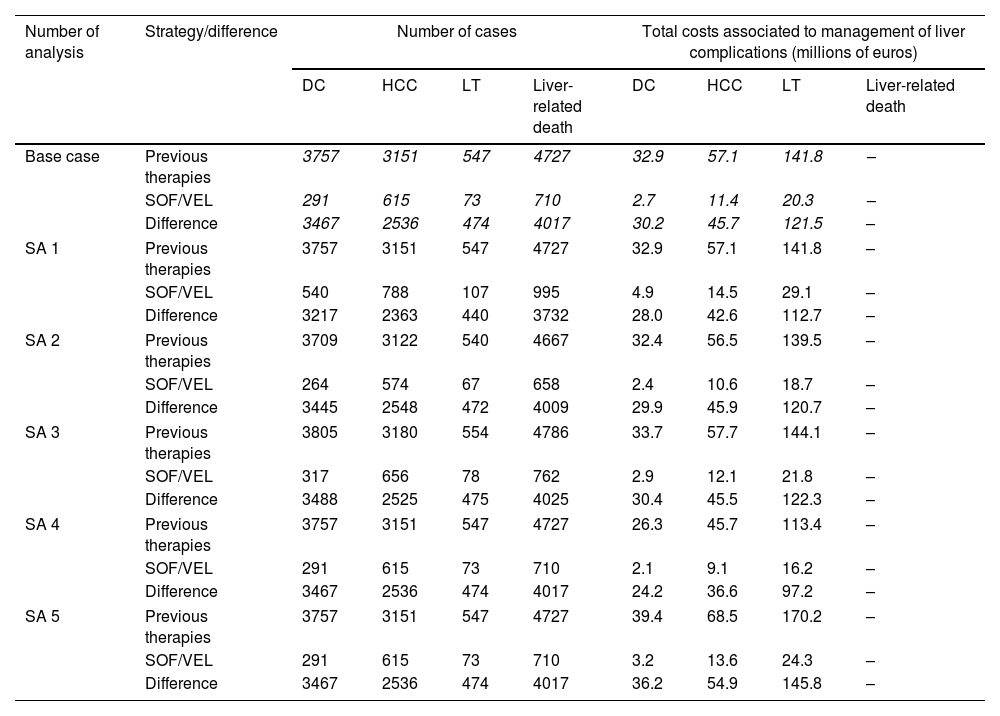
Different sensitivity analyses were performed varying the SVR rate from SOF/VEL to 95%, the percentage of patients with cirrhosis (±10%) and the cost associated with the management of liver complications states (±20%).
ResultsThe clinical results showed that treatment with SOF/VEL reduced, throughout the life of the patients, mortality due to liver disease and clinical events of the liver by 85% (92% decompensated cirrhosis, 80% hepatocellular carcinomas and 87% liver transplants) compared to previous therapies (Fig. 1).
The economic results showed that treatment with SOF/VEL compared to previous therapies yields a savings of €197 million associated with the management of clinical liver events. The greatest impact was on the cost of managing decompensated cirrhosis (decrease of 92%), followed by liver transplants (decrease of 87%) and hepatocellular carcinoma (decrease of 80%) (Fig. 2).
The sensitivity analysis showed that even under the assumption of a lower SVR rate (94.6%), SOF/VEL would have a significant impact on clinical and economic outcomes, reducing the number of liver deaths by 79%, the number of clinical events similarly (86% decompensated cirrhosis, 75% hepatocellular carcinomas, and 80% liver transplants), and the total cost associated with the events by €183 million. Furthermore, a decrease or increase in the number of patients with cirrhosis would cause a decrease between 82 and 93% and an increase between 79 and 92% in the number of cases of liver complications and liver mortality, respectively, also causing a fluctuation in the costs in line with the variation. Similarly, this variation also occurred when states’ costs were modified by 20%, not affecting the difference in the number of cases (Table 1).
Sensibility analysis: number of cases with each treatment and their costs.
| Number of analysis | Strategy/difference | Number of cases | Total costs associated to management of liver complications (millions of euros) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DC | HCC | LT | Liver-related death | DC | HCC | LT | Liver-related death | ||
| Base case | Previous therapies | 3757 | 3151 | 547 | 4727 | 32.9 | 57.1 | 141.8 | – |
| SOF/VEL | 291 | 615 | 73 | 710 | 2.7 | 11.4 | 20.3 | – | |
| Difference | 3467 | 2536 | 474 | 4017 | 30.2 | 45.7 | 121.5 | – | |
| SA 1 | Previous therapies | 3757 | 3151 | 547 | 4727 | 32.9 | 57.1 | 141.8 | – |
| SOF/VEL | 540 | 788 | 107 | 995 | 4.9 | 14.5 | 29.1 | – | |
| Difference | 3217 | 2363 | 440 | 3732 | 28.0 | 42.6 | 112.7 | – | |
| SA 2 | Previous therapies | 3709 | 3122 | 540 | 4667 | 32.4 | 56.5 | 139.5 | – |
| SOF/VEL | 264 | 574 | 67 | 658 | 2.4 | 10.6 | 18.7 | – | |
| Difference | 3445 | 2548 | 472 | 4009 | 29.9 | 45.9 | 120.7 | – | |
| SA 3 | Previous therapies | 3805 | 3180 | 554 | 4786 | 33.7 | 57.7 | 144.1 | – |
| SOF/VEL | 317 | 656 | 78 | 762 | 2.9 | 12.1 | 21.8 | – | |
| Difference | 3488 | 2525 | 475 | 4025 | 30.4 | 45.5 | 122.3 | – | |
| SA 4 | Previous therapies | 3757 | 3151 | 547 | 4727 | 26.3 | 45.7 | 113.4 | – |
| SOF/VEL | 291 | 615 | 73 | 710 | 2.1 | 9.1 | 16.2 | – | |
| Difference | 3467 | 2536 | 474 | 4017 | 24.2 | 36.6 | 97.2 | – | |
| SA 5 | Previous therapies | 3757 | 3151 | 547 | 4727 | 39.4 | 68.5 | 170.2 | – |
| SOF/VEL | 291 | 615 | 73 | 710 | 3.2 | 13.6 | 24.3 | – | |
| Difference | 3467 | 2536 | 474 | 4017 | 36.2 | 54.9 | 145.8 | – | |
SA1: SVR rate from SOF/VEL to 95%; SA2: percentage of patients with cirrhosis (−10%); SA3: percentage of patients with cirrhosis (+10%); SA: cost associated with the management of liver complications states (−20%); SA5: cost associated with the management of liver complications states (+20%); SA, sensitivity analysis; DC, decompensated cirrhosis; HCC, hepatocellular carcinoma; LT, liver transplant.
DAAs have made it possible to achieve SVR rates much higher than those of previous therapies, close to 98%,11 through which they have changed the evolution of chronic hepatitis C, improving survival and the clinical and economic burden of the disease. In our analysis, this impact was estimated in a cohort of patients treated with a pangenotypic DAA during the last 5 years. The results showed that, compared to previous therapies, SOF/VEL significantly reduced mortality and clinical events associated with chronic hepatitis C, in addition to reducing the cost of managing these events, representing significant savings to the NHS. These results are in line with a similar analysis of the impact of sofosbuvir-based regimens in Spanish patients.15 Our results also show a decrease in the prevalence of liver events evidenced in other Spanish studies since the arrival of the new antivirals. These studies show a significant decrease in cases of cirrhosis, leading to a decrease in hospitalizations, from 49% in 2014 to 27% in 201921 and hepatocellular carcinoma (decrease in cases: 79% in 2011–2005 to 55% in 2015–2020),22 although the risk of development hepatocellular carcinoma persists after the treatment response in patients with fibrosis or cirrhosis,23,24 a risk that was considered in the model. The impact of the use of SOF/VEL in decompensated cirrhosis has also been reflected in other studies25 as well as in our results. Likewise, recent studies have shown a great slowdown in HCV mortality, especially associated with hepatocellular carcinoma, after the advent of DAAs.26,27 This trend is also reflected in our analysis, resulting in a significantly lower number of liver deaths when treating with SOF/VEL than with previous therapies.
In economic terms, DAAs have also had an economic impact on the health system related to the evolution of HCV disease.13,15,28 From this perspective, our analysis estimated substantial savings in the management of liver disease when comparing the use of SOF/VEL with previous therapies, which contributes to the sustainability of the NHS. In the same way, and although they have not been considered in the analysis, some studies that demonstrate an association between SVR and a lower risk of extrahepatic manifestations7 and, consequently, in the consumption of health resources to manage them. Although these costs have not been considered here, they are a substantial part of the economic burden of the disease, and any improvement associated with them should be considered when evaluating the efficiency of the new treatments.
The SVR rate, nearly 99%, that we assumed for the SOF/VEL cohort was taken from a real-life study with a representative sample of patients. Even so, due to the heterogeneity between patients, a sensitivity analysis was done in which we reduced the SVR rate to 95%. This change had an incremental impact on the incremental, showing fewer events and lower costs than in the base case. Even so, the differences concerning the use of previous therapies are still quite remarkable. Likewise, because patients in advanced stages of fibrosis progress earlier to more serious states such as decompensated cirrhosis and hepatocellular carcinoma, different sensitivity analyses were performed varying the percentage of cirrhotic patients, in addition to the cost associated with each of them and the progression to liver transplant. These analyses showed an impact on the results in line with the variation, increasing or decreasing clinical events and costs, respectively.
A limitation of the current study is that the analysis focused only on estimating the clinical and economic benefits of SOF/VEL, without considering other treatments used in the management of chronic hepatitis C. However, our objective was to highlight what has been achieved clinically and economically with Sofosbuvir, the first pangenotypic AAD in the therapeutic arsenal for hepatitis C, which continues with SOF/VEL a treatment regimen that has represented a transformation in the Treatment of these patients.
On the other hand, the scientific evidence shows that the benefits of achieving SVR with DAAs are multiple, not only for the health system, and should be considered when evaluating DAA treatments. These benefits are the improvement in the quality of life that comes from reducing the burden of the disease, especially in patients with cirrhosis and decompensation,29-32 and the social gains from the indirect costs that are no longer incurred from the loss of productivity due to disease progression.33
In addition, the advent of DAAs, and especially access to treatment for those patients who were limited by previous therapies, has changed the approach to HCV disease, facilitating the eradication of hepatitis C. SOF/VEL is accessible to most patients without restrictions, in addition to being a pangenotypic treatment, helping to simplify the diagnosis of the disease by eliminating the test and the RNA follow-up visits at 4 weeks and at the end of the treatment.
Finally, we emphasize that the objective of elimination would not be possible without the implementation of actions planned and undertaken by multidisciplinary teams focussed on the detection and diagnosis of HCV-infected people to support their access to treatment.
ConclusionsBased on the economic model developed to evaluate the value of SOF/VEL for hepatitis C treatment, SOF/VEL reduces the clinical and economic burden of the disease and contributing to the goal of eliminating the disease in Spain.
AuthorshipRDH and MAC adapted the model, reviewed the scientific literature, performed the analyses and drafted the manuscript. RDH, MAC, RE and HC validated the model structure and the inputs and provided information about the clinical management of hepatitis C patients receiving SOF/VEL in Spain. All the authors contributed to interpretation of the results and reviewed and approved the final version of the manuscript.
Artificial intelligence (AI)During the preparation of this paper, the author(s) didn’t used of AI and AI-assisted technologies in the writing process in their manuscript.
FundingThe study was funded by Gilead Sciences Spain.
Conflict of interestRE speaker and advisory AbbVie and Gilead.
RDH and MAC are employees of Pharmacoeconomics & Outcomes Research Iberia (PORIB), a consulting firm specializing in the economic evaluation of health care interventions that has received financing from Gilead Sciences Spain for the development of the project that is not conditional upon the results.
HC is an employee of Gilead Sciences Spain and has declared that no competing interests exist.