Anti-tumour necrosis factor α therapy in inflammatory bowel disease has been shown to be effective in clinical practice.
After more than a decade using these therapies the question arises about whether there is an appropriate time to suspend these therapies, and how this should be done. This review aims to evaluate the current evidence on these topics concerning anti-tumour necrosis factor α therapies, and eventually identify conditions and subgroups of patients that could potentially be candidates for withdrawal.
La efectividad de la terapia con fármacos antifactor de necrosis tumoral α en la enfermedad inflamatoria intestinal ha sido probada en la práctica clínica.
Tras más de una década de uso de este tipo de fármacos, surge la interrogante acerca de si existe un momento apropiado para suspender estas terapias, y de qué manera esta debiera realizarse. Esta revisión tiene por objetivo evaluar la evidencia actual acerca del retiro de la terapia con antifactor de necrosis tumoral α y, eventualmente, identificar las condiciones o los subgrupos de pacientes potencialmente candidatos a la suspensión o el retiro de esta.
Due to its gastrointestinal and extraintestinal manifestations, inflammatory bowel disease (IBD) affects sufferers’ functionality and quality of life in many ways. The aim of treatment is to achieve deep remission of the disease (clinical remission with mucosal healing and histology without signs of acute activity), while also aiming to minimise the risks associated with the therapy.1 Treatment in IBD needs to be long term, with frequent medical and laboratory follow-up. Of the different treatments used, the introduction of biological therapy marked a turning point in the management of patients with inflammatory activity not controlled by traditional treatments such as 5-aminosalicylates, corticosteroids and immunosuppressants (IS) (methotrexate and thiopurines).
The classic approach of step-up therapy has neither proven to be effective in all patients nor does it alter the natural history of the disease.2,3 This has been changing with the early indication of IS and/or biological agents, seeking to rapidly control the inflammatory activity with effective, short-term adjustments to treatment.4,5 However, in addition to its high cost,8 this approach involves potential risks, including the onset of infections, lymphoproliferative disorders and some neoplasms such as skin cancer.6,7 Consequently, in patients who are in deep remission for a prolonged period and who have a low risk of relapse, the possibility of reducing the dose of the biological drug to a minimum has been raised, increasing the interdose interval and eventually stopping the treatment altogether. This strategy has not yet been accepted as routine practice. A recent survey of 182 gastroenterologists from the United States and 127 from Europe showed that the European group was more predisposed to discontinuing one of the combination therapy drugs (biological drug+IS) in patients with IBD who were in remission (44% vs. 18%).9 The patients themselves have different positions on the subject. In a survey which only included patients with Crohn's disease (CD), the French group considered themselves more likely to discontinue combination therapy than the American group (69% vs. 48%, p<0.01).10
The aim of this review is to assess the evidence and propose a strategy of when, how and in which patients biological therapy and/or IS could be de-escalated and/or withdrawn, while also addressing the risk of relapse associated with such a decision.
Withdrawal of biological therapy in inflammatory bowel diseaseAnti-tumour necrosis factor-α (anti-TNF-α) subclass IgG1, infliximab (IFX), a chimeric monoclonal antibody, and adalimumab (ADA), a humanised monoclonal antibody, have been shown to be effective as induction and maintenance therapy in both ulcerative colitis (UC) and CD.11–15 The mucosal healing rates achieved by IFX and ADA are around 30% in CD and 60% in UC, after the induction phase, and 25–40% for CD and 30–35% for UC in the maintenance phase (weeks 52–54).16,17 “Real life” studies show higher rates of treatment response, reaching up to 90% in the induction phase and 56% in follow-up at 28 months,18 with anti-TNF-α failure-free survival in 65.9% at five years.19
Combination therapyWithdrawal/de-escalation of immunosuppressants in combination therapy with anti-TNF-αThe formation of anti-IFX antibodies during therapy can affect 43% (with IS) to 75% (without IS) after the fifth infusion of IFX.20 This leads to a decrease in plasma levels (PL) of IFX and loss of effectiveness of the drug (immunogenic failure). To avoid the above, combination therapy with IS, thiopurines or methotrexate would be indicated at least in the first period of IFX use,21 as it is a strategy which, on the one hand, decreases the immunogenicity of IFX and on the other, increases the circulating PL of the biological agent.22
In the case of ADA, since it is a humanised antibody, it is expected to have a lower associated rate of antibody formation, which means the IS would exert less of an effect. A recent study shows that combing thiopurines with ADA would not result in less formation of anti-ADA antibodies vs. ADA in monotherapy (26 vs. 28%, p=1.00).23
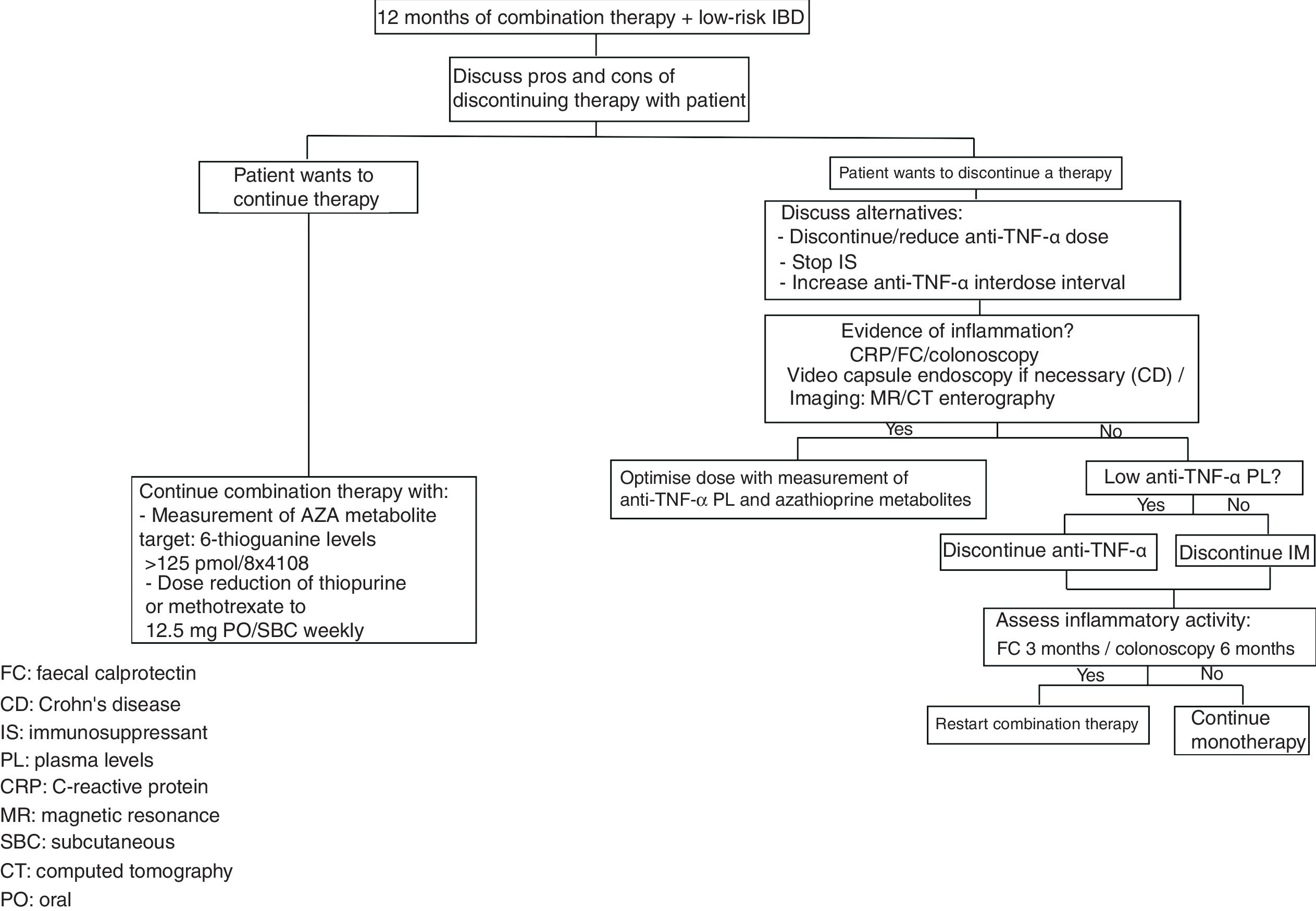
Crohn's diseaseIn CD there is generally no significant difference between continuing or withdrawing IS when using biological therapy as the background treatment, with both groups showing a high response rate during follow-up in the first year after withdrawal, but a decrease in response rate in subsequent years. This simply confirms the need to perform clinical, biochemical (faecal calprotectin [FC]), endoscopic and imaging follow-up as required.
A study by Van Assche et al. included 80 patients with CD in clinical remission who were on combination therapy for at least six months (IFX 5mg/kg every 8 weeks combined with IS, either azathioprine [AZA], 6-mercaptopurine or methotrexate). These patients were randomised to continue (n=40) or discontinue (n=40) the IS. No difference was found between the two groups when comparing the need to stop or shorten the IFX administration intervals (p=0.65). However, C-reactive protein (CRP) values were higher in the group that discontinued than in the group that continued taking the IS (2.8 vs. 1.6mg/l; p<0.005). PL of IFX were lower in the group that discontinued the IS (1.65 vs. 2.87μg/ml; p<0.0001). In a follow-up period of 104 weeks, discontinuation did not lead to a higher relapse rate compared to the group that continued taking the IS.24
A retrospective study assessed 48 patients with CD on combined treatment with AZA and IFX for at least six months (median 30.2±17.3 months) whose AZA was discontinued once they were in clinical remission. 13 patients (27%) required a change in therapeutic strategy in the subsequent 14±6.4 months: in nine because of reactivation, requiring infusion intervals to be shortened to every six weeks; three suffered severe intolerance to IFX and had to change to ADA; and one patient required surgery for ileal stenosis. At the end of follow-up, 35/48 (73%) were relapse-free, with no need for corticosteroids or IS, with the likelihood of relapse-free survival on monotherapy with IFX being 85% (±5%) at 12 months and 41% (±18%) at 32 months.25
No studies were found to have specifically assessed outcomes in patients on treatment with combination therapy after withdrawal of methotrexate treatment.
Ulcerative colitisIn UC, this situation has only been assessed in one retrospective study of 82 biological therapy-naïve patients, with a primary response to induction with IFX, on combination therapy with AZA for at least six months, and in prolonged steroid-free remission (blood-free stools and no increase in stool frequency). These patients were followed up for a median time of 22.3±14 months, which was divided into quarters. Treatment was continued with IFX and AZA, with the AZA being discontinued at the doctor's discretion (sum of 393 quarters with combination therapy and 282 quarters with IFX monotherapy). Patients in the combination therapy group had fewer relapses (12/393 vs. 33/282 quarters, p=0.049) and the time to relapse was longer (16.6 vs. 7 months; p<0.05) than in the monotherapy group. A combination therapy duration longer than nine months had an inverse association with clinical relapse (HR=0.32, 95% CI 0.15–0.70, p=0.004). The authors propose that in UC, the combination therapy regimen should be maintained for at least nine months before AZA is withdrawn.26
One alternative to discontinuing the IS in patients with CD and UC is to lower the dose, in order to reduce the risk of adverse events while maintaining its contribution to the effectiveness of the anti-TNF-α. Levels of 6-thioguanine >125pmol/8×108 would be sufficient to maintain adequate PL of IFX.27 With methotrexate, doses of >12.5mg/week would be more effective in maintaining the response to combination therapy.28
Withdrawal of anti-TNF-α in combination therapy with immunosuppressantsThe STORI trial was a prospective multicentre study on 115 patients with luminal CD who were treated for at least 12 months with IFX in combination with IS, and were in steroid-free remission for at least six months before the withdrawal of IFX. Relapse rates at 12 and 24 months were 43.9%±5% and 52.2%±5.2%, respectively, with a median time to relapse of 16.4 months. The risk factors that may have been associated with a greater risk of relapse before the withdrawal of IFX were: being male; no surgical resection; white blood cell count >6000/l; haemoglobin <145g/l; CRP >5mg/l; and FC >300μg/g. Patients with ≤2 risk factors (29% of the population studied) had a 12-month relapse risk of 15%, while 88% of patients who relapsed had a favourable clinical response on restarting treatment with IFX.29
Of the 102 patients followed up in this study, after seven years 21.6% had not needed to restart biological therapy and had not suffered any major complications. Of those who did restart IFX, it had failed in 30.1% after six years. The authors also found that 70.2% of the patients had no problems upon withdrawal of the therapy (no major complications or failure of retreatment with IFX).30
Papamichael et al. conducted a retrospective study of 100 patients with CD who discontinued IFX (median treatment time prior to withdrawal of 7.3 months) after achieving clinical remission (clinical inactivity with Harvey-Bradshaw Index [HBI] <4). 84 patients continued on IS, 16 on 5-ASA and five had no other associated therapy. They were followed up for a median time of 9.7 years (8–11.5 years). At the end of follow-up, 52% of the patients showed sustained clinical remission, defined as maintenance of disease remission, without the need to escalate therapy or for CD-related surgery, until the end of the follow-up period. A higher likelihood of sustained clinical remission was associated with an age at diagnosis >25 years (p=0.012) and less than one year since disease onset (p=0.017) and of the parameters measured at the time of withdrawal, PL of IFX <6μg/ml (p=0.031), mucosal healing (p=0.046) and vascular cell adhesion molecule-1 (VCAM-1) (+) (p=0.024).31
A recent multicentre, retrospective, observational study assessed 1055 patients with IBD (731 CD and 324 UC) being treated with anti-TNF-α (781 with IFX and 274 with ADA) who then had the biological drug withdrawn (75% electively, 18% for adverse events and 7% for remission after the top-down strategy). The median follow-up after withdrawal was 19 months (range: 6–176). After the withdrawal, 689 patients continued taking thiopurines, 29 methotrexate, 1495-ASA and 188 were on no treatment. The relapse rate was 19% per patient-year in CD and 17% per patient-year in UC (95% CI 15–20; p=0.1). The multivariate analysis showed a higher risk of relapse in patients previously treated with ADA compared to IFX (HR=1.29; 95% CI 1.03–1.6; p=0.027) and in patients who electively stopped taking anti-TNF-α because of an adverse event compared to those who stopped after the top-down strategy (HR=1.82; 95% CI 1.19–2.79; p=0.006 and HR=1.95; 95% CI 1.22–3.12; p=0.005, respectively). A lower risk of relapse was associated with continuing IS therapy (HR=0.99; 95% CI 0.98–0.99; p=0.002) and being older when the drug was discontinued (HR=0.7; 95% CI 0.57–0.88; p<0.0001).32
A systematic review and meta-analysis of 27 studies (21 with IFX and 6 with IFX or ADA) in patients with CD and UC which assessed the risk of relapse after withdrawal of anti-TNF-α reported that the overall risk of relapse was 44% in CD and 38% in UC. Analysis of the relapse rate 12 months after withdrawal of the therapy showed relapse rates at 12 and >25 months of 40% and 49% respectively for CD, while for UC, the relapse rates at 12 and 24 months were 28% and 36% respectively. After restarting therapy with anti-TNF-α, induction of remission was achieved in 80% of patients.33 One study prospectively followed 78 patients (CD=61, UC=17) for a median of 30 months (7–47 months), who had their anti-TNF-α therapy discontinued after achieving endoscopic remission (absence of ulcerations). A cumulative 24-month relapse rate of 49% was found, with no differences between CD and UC (p=0.63). A subanalysis that compared patients in endoscopic and biochemical remission (FC<150μg/g+CRP<5mg/l) (n=27) to patients in clinical and endoscopic remission (n=23) found 24-month relapse rates of 60% and 52% respectively (p=0.84).34
Although methodologically the above studies are quite heterogeneous (different definitions of clinical remission and time in remission prior to withdrawal), the relapse rates are similar after stopping anti-TNF-α therapy, with previous combination therapy, ranging from 21% to 56% at 12 months, and from 47% to 64% at 24 months. This shows that patients who are in clinical and endoscopic remission may be able to stop taking the biological therapy, although this option needs to be assessed on an individual basis and discussed with the patient.
Withdrawal of anti-TNF-α monotherapyThere is insufficient evidence on the withdrawal of anti-TNF-α in monotherapy. The majority of the studies involve patients on combination therapy in whom one of the two treatments is discontinued.
Crohn's diseaseThe abovementioned Casanova et al. study shows that the cumulative relapse rate post-withdrawal of anti-TNF-α was 44% per patient-year, with no differences between CD and UC (p=0.1). However, the analysis looking at the withdrawal from combination therapy vs. monotherapy found the incidence of relapse in patients who continued on treatment with AZA to be 17% per patient-year compared to 26% per patient-year in patients without IS.32
Another study evaluated the time interval between IFX withdrawal and clinical relapse in 46 patients with CD; 23 with luminal CD with a good response to three induction doses of IFX, and 23 with luminal CD (n=11) or perianal CD (n=12) with a sustained response to maintenance therapy with IFX every eight weeks, in remission for at least 12 months. In the patients with perianal disease, the cumulative probability of remaining relapse-free after the withdrawal of IFX was 45% at six months and 34% at 12 months, and in patients with luminal disease, 83% at 12 months.35 However, it would seem that, despite a tendency towards lower relapse rates when immunosuppression is maintained after the withdrawal of biological therapy, there is insufficient evidence to date to either support or rule out such a pattern in luminal CD. In the case of perianal CD, there is clearer evidence that the withdrawal of IFX is not advisable, in view of the high relapse rate in the short term.
In this same context, the multicentre, prospective, observational RASH study assessed 121 patients with CD in clinical remission (CDAI<150) after 12 months on IFX (n=87) or ADA (n=34) who had the therapy discontinued. The relapse rate at 12 months was 45%, with a mean time to relapse of six months (3.75–12 months). Previous biological therapy use (one year apart) and the need to use an intensified regimen during the year of therapy were identified as risk factors associated with relapse.36
Ulcerative colitisIn UC, after the withdrawal of maintenance biological therapy, the relapse rate is almost three times higher than among patients who continue the therapy. However, these data are based on retrospective studies that only used clinical criteria and did not use biomarkers or consider current standards for remission which include biochemical, endoscopic and histological variables.
In a retrospective study of 193 patients with UC treated with IFX, the withdrawal of biological therapy was assessed in 111 who were in clinical remission for at least 12 months before withdrawal, without concomitant corticosteroid therapy or immunosuppression. The remaining 82 patients continued on IFX therapy. The relapse rates in those who discontinued the therapy compared to those who continued it were 23.3 vs. 7.2 per 100 person-years. Of the 53 patients who relapsed after the withdrawal of IFX, 66% restarted therapy with the same biological drug (the remaining 44% were treated with corticosteroids, ADA or golimumab). After IFX was restarted, the response rate (decrease of at least three points or 30% from baseline in the Mayo score) reached 77.1%, and the remission rate, 51.4%.37
Measurement of anti-TNF-α PL for withdrawal of therapyOne way to assess the possible withdrawal of biological therapy is to measure the PL of the biological agent. A retrospective study assessed 48 patients (30 CD, 18 UC) who stopped anti-TNF-α therapy (35 IFX/13 ADA) while in remission, having been on treatment for an average of 22.7±12.4 months, and undergone measurement of the anti-TNF-α PL. Before the withdrawal 40/42 (95%) patients had an endoscopy showing no signs of activity and 31/48 (65%) had CRP and FC (<50μg/g) within normal ranges. At the time of the withdrawal of anti-TNF-α, 20 patients had detectable PL and 28 undetectable PL of the biological drug. The number of patients who relapsed was greater in the group with detectable PL (16/20 vs. 9/28) (OR 8.4, 95% CI 2.2–32; p=0.002). Similarly, relapse-free survival after the withdrawal of anti-TNF-α was significantly higher in patients with undetectable PL (p<0.001). It is important to note that 14 patients (29%) were on concomitant IS therapy when the biological drug was discontinued, eight of whom relapsed during follow-up after withdrawal.38
The measurement of PL could have a role in the biological therapy withdrawal strategy, helping to determine whether it is possible to discontinue the IS or the anti-TNF-α. However, it is important to recognise that the utility of PL in this scenario has not been assessed prospectively.
Strategies: anti-TNF-α dose reduction and increased inter-dose intervalBoth decreasing the dose of anti-TNF-α or lengthening the interval between doses have been assessed in a number of studies. One study prospectively assessed a cohort of 12 patients with CD treated with IFX (induction and maintenance [5mg/kg]) in the two weeks following a CD-related intestinal resection. These patients had no endoscopic activity 24 months after surgery and were followed up for a further 12 months, after which the drug was discontinued without starting any other therapy. Colonoscopy at week 16 post-withdrawal showed 10/12 (82%) patients to have endoscopic recurrence (Rutgeerts score >2, disregarding lesions around the anastomosis), eight of whom had a Rutgeerts score of 3. They were given IFX 1mg/kg to start with, with no mucosal improvement after three infusions. The dose was then increased to 2mg/kg and repeat colonoscopy after the third infusion showed that all of the patients’ scores had decreased by one point (p=0.006), although the average Rutgeerts score remained at 2. The dose was therefore increased again to 3mg/kg, at which mucosal integrity was restored (Rutgeerts score of 0 or 1). Endoscopic remission was sustained at week 52 of follow-up with an IFX dose of 3mg/kg every eight weeks.39
A retrospective cohort case-control study compared 40 patients with CD on treatment with ADA every two weeks (median of 17.9 months) and PL of ADA >7μg/ml to 40 patients with CD on treatment with ADA in monotherapy (median of 43 months), in whom it was decided to lengthen the ADA dose interval to every three weeks due to adverse events (n=1), ADA PL >7μg/ml (n=8) or both (n=31). In both groups, ADA PL were available. At two years of follow-up, 65% maintained a clinical response. The remaining 35% required escalation back to the previous dose due to clinical relapse, low ADA PL or both. There was a reduction in adverse events in the group in whom the ADA dose interval was lengthened (55% vs. 100%, p<0.001). Having CRP <3.5mg/l at dose de-escalation was associated with longer dose escalation-free survival (OR=6.28, 95% CI 1.83–21.59, p=0.004).40
As far as maintenance dose de-escalation is concerned, the study by Sorrentino et al.39 shows that with IFX 3mg/kg every eight weeks, patients would achieve an adequate endoscopic response. However, this optimisation strategy lacks measurement of drug PL, which would be a useful tool for personalising the dose change. In terms of the dose interval, the study by Van Steenbergen et al.40 shows that there are patients in whom the interval for administering the biological drug can be lengthened. This would involve measuring the drug PL in conjunction with inflammatory markers which would guide us with greater certainty as regards the patient's progress, albeit indirectly.
In this context, the TAXIT study showed that patients treated with IFX with PL >7μg/ml could reduce the dose of the drug without losing their response, resulting in more efficient use and a cost reduction of 28% compared to before the dose reduction (p<0.001).41
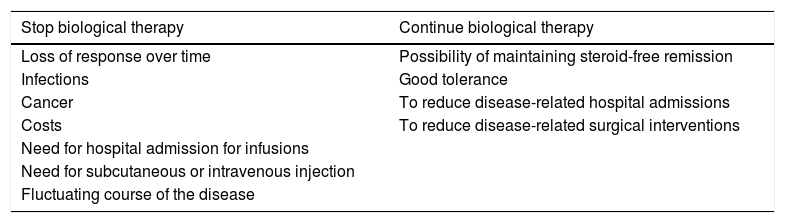
DiscussionBiological agents provide an established therapeutic benefit in the management of IBD. However, in view of their long-term safety profile, high associated costs and the natural history of IBD (activation-remission), consideration is now given to the possibility of withdrawing therapy at some point (Table 1). In a recent survey of gastroenterologists in Europe and the United States, the main reasons for discontinuing IS treatment and biological therapy in patients in remission were fear of the risk of cancer and financial cost, respectively.9
Reasons to continue or discontinue biological therapy.
| Stop biological therapy | Continue biological therapy |
|---|---|
| Loss of response over time | Possibility of maintaining steroid-free remission |
| Infections | Good tolerance |
| Cancer | To reduce disease-related hospital admissions |
| Costs | To reduce disease-related surgical interventions |
| Need for hospital admission for infusions | |
| Need for subcutaneous or intravenous injection | |
| Fluctuating course of the disease |
Some have suggested discontinuing the therapy for a while, with the option to restart it as required (elevated FC and/or CRP).42 Obviously, given the risk of losing anti-TNF-α efficacy after restarting, this strategy would be safer if there were greater access to new drugs (anti-integrins, anti-p40 IL-12/23, Janus kinase [JAK] inhibitors).
When deciding to reduce the dose or discontinue a biological agent, strict patient monitoring is required in order to detect early relapses. However, the form and frequency of this follow-up are yet to be defined. In general, different studies34,38,40,42 have considered clinical and biochemical follow-up with CRP and FC every 8–12 weeks on average, with the aim being that clinical relapse could be predicted in advance. This would enable an early endoscopic or radiological approach in the event of marked elevation of these parameters.
In terms of the risk factors associated with a higher relapse rate after withdrawal of anti-TNF-α therapy, several studies show that evidence of inflammation when the therapy is stopped, be that elevated CRP and/or FC or a lack of mucosal healing, is associated with a higher relapse rate. This supports the idea that a patient needs to be in deep remission before withdrawal of an anti-TNF-α can be considered.26,32,38
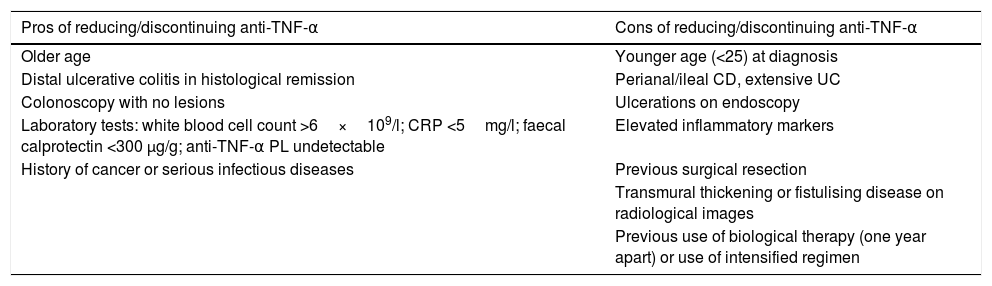
There are risk factors and protective factors to consider before the withdrawal of biological therapy (Table 2) and the decision to discontinue/reduce treatment therefore has to be made on an individual basis, with these patients followed up closely with laboratory tests and monitoring of preventive measures (Fig. 1).
Pros and cons of reducing/discontinuing anti-TNF-α biological therapy.
| Pros of reducing/discontinuing anti-TNF-α | Cons of reducing/discontinuing anti-TNF-α |
|---|---|
| Older age | Younger age (<25) at diagnosis |
| Distal ulcerative colitis in histological remission | Perianal/ileal CD, extensive UC |
| Colonoscopy with no lesions | Ulcerations on endoscopy |
| Laboratory tests: white blood cell count >6×109/l; CRP <5mg/l; faecal calprotectin <300 μg/g; anti-TNF-α PL undetectable | Elevated inflammatory markers |
| History of cancer or serious infectious diseases | Previous surgical resection |
| Transmural thickening or fistulising disease on radiological images | |
| Previous use of biological therapy (one year apart) or use of intensified regimen |
In conclusion, the evidence on the utility of biological therapy in IBD is clear, but the point at which it may be discontinued or withdrawn is still yet to be established. Prospective studies assessing this strategy and which include the new molecules being proposed in IBD management will enable us to define the best scenario for each patient.43
Conflict of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Sedano Muñoz R, Quera Pino R, Lubascher Correa J, Pizarro Jofré G, Simian Marín D. Evaluación del retiro progresivo y/o la suspensión de la terapia anti-TNF-α en la enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2019;42:133–140.